|
Case Report
Robotic surgical resection of a pancreatic dermoid cyst: Case report and review of literature of an extremely rare pancreatic neoplasm
1 Department of General Surgery, AdventHealth Orlando, 2415 N Orange Avenue, STE 400, Orlando, FL 32804, USA
2 Ross University School of Medicine, Bridgetown, Barbados
3 Department of Pathology, AdventHealth Orlando, 2415 N Orange Avenue, STE 400, Orlando, FL 32804, USA
4 Department of Surgical Oncology, AdventHealth Cancer Institute, Orlando, FL 32804, USA
Address correspondence to:
William Cobb
MD, 2415 N Orange Avenue, STE 400, Orlando, FL 32804,
USA
Message to Corresponding Author
Article ID: 100020G01WC2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Cobb W, Marks J, Tran TA, Rosales A. Robotic surgical resection of a pancreatic dermoid cyst: Case report and review of literature of an extremely rare pancreatic neoplasm. Edorium J Gastroenterol 2025;10(1):11–16.ABSTRACT
Introduction: Mature cystic teratomas, also called dermoid cysts, are benign, well-differentiated tumors originating from cells of all three dermal layers. Pancreatic dermoid cysts are extremely rare with the first published case by Kerr in 1918 and only 55 subsequent published cases since. Literature review was conducted using PubMed, Ovid, Clinical Key, Cochrane Library, Web of Science, and UpToDate; results were not limited by publication date. We reviewed the resulting literature to determine the incidence and prior reported cases, pathology, presentation, imaging findings, and management to previous literature.
Case Report: Here we present the case of a 72-year-old male with history of left nephrectomy for a benign kidney cyst that during surveillance imaging, a pancreatic mass was identified. He was referred to us for this incidental finding of a possible pancreatic tail cyst that increased in size during surveillance. Computed tomography (CT) scan revealed a cystic focus at the pancreatic tail measuring 5.1 × 3.6 cm. Endoscopic ultrasound (EUS) revealed an anechoic lesion suggestive of a cyst with an overall impression of an intraductal papillary mucinous neoplasm-branch duct (IPMN-BD). Further fine-needle aspiration (FNA) pathology revealed amorphous material consistent with cyst contents and no epithelial cells identified. The patient underwent a robotic-assisted distal pancreatectomy with splenectomy. Pathology was consistent with a 5.5 cm dermoid cyst of the pancreas, negative for malignancy. We present a case of robotic-assisted surgical intervention for pancreatic tail dermoid cyst.
Conclusion: Our case presents the first detailed report of a robotic distal pancreatectomy with splenectomy for dermoid cysts of the pancreas with prior retroperitoneal surgery. Robotic distal pancreatectomy with splenectomy is safe and feasible in patients with cystic lesions and prior history of ipsilateral retroperitoneal surgery. Further research should focus on pre-operative workup for proper diagnosis and further identification of incidence and prevalence.
Keywords: Dermoid cyst, Mature cystic teratoma, Pancreas, Surgical oncology
INTRODUCTION
Pancreatic dermoid cysts are extremely rare and the first published case was by Kerr in 1918 [1]. Dermoid cysts, also called cystic teratomas, are congenital germ cell tumors that originate from totipotent stem cells and include all three dermal layers, endoderm, mesoderm, and ectoderm. They are classified as either mature or immature based on the neuroectodermal elements within the tumor [2],[3],[4],[5]. Mature cystic teratomas, also called dermoid cysts, are benign, well-differentiated tumors that are more commonly encountered in the ovaries and testes [6],[7]. Since 1918, there are only 55 additional published cases, with about 15% reported in the tail of the pancreas [8]. These tumors present a diagnostic challenge as they commonly present with generalized, nonspecific symptoms and their radiologic appearance is largely dependent on their composition. Though malignant degeneration of pancreatic dermoid cysts has never been reported in the literature, there are reports of other teratomas with malignant potential. Thus, accurate diagnosis and successful treatment remains of vital importance even with a lack of consensus of definitive preoperative diagnostic testing or pathognomonic findings.
CASE REPORT
Our patient is a 72-year-old male with a past medical history of rheumatoid arthritis on certolizumab pegol, hypertension, a benign cyst of the left kidney status post left nephrectomy, and asymptomatic duodenal diverticulum originally referred to us for incidental findings of a pancreatic tail cyst during recent hospitalization for diverticulitis. Prior to recent hospitalization, the patient denied any abdominal pain, nausea, vomiting, diarrhea, constipation, bloating, fullness, pancreatic exocrine insufficiency symptoms, or episodes of pancreatitis.
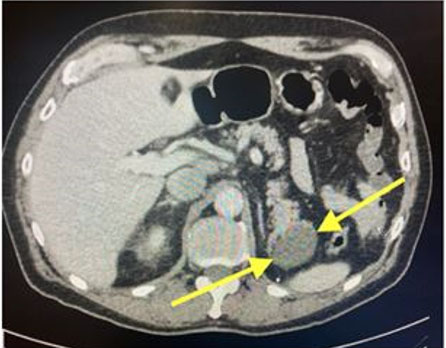
An abdominopelvic computed tomography (CT) scan revealed a cystic focus at the pancreatic tail measuring 5.1 × 3.6 cm with no main duct dilatation or pancreatic fluid collections (Figure 1).
This was followed by an endoscopic ultrasound (EUS) that revealed an anechoic lesion suggestive of a cyst identified in the pancreatic tail with an overall impression of an intraductal papillary mucinous neoplasm-branch duct (IPMN-BD). Fine-needle aspiration (FNA) pathology was suggestive of amorphous material consistent with cyst contents and no lining epithelial cells were identified.
Lab work included a carcinoembryonic AG (CEA) < 2.0 ng/mL, cancer antigen 19-9 (CA 19-9) of 489 U/mL (elevated), amylase of 57 U/L, and a total prostate-specific antigen (PSA) of 4.57 ng/mL. Liver enzymes were all within normal limits.
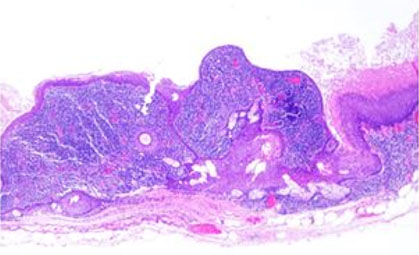
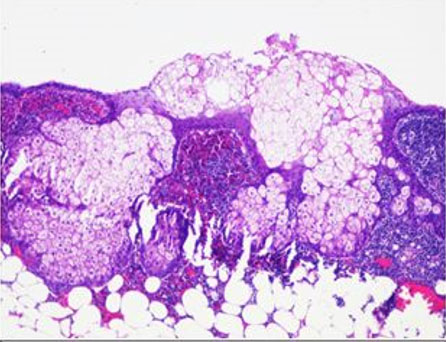
Robotic-assisted distal pancreatectomy and splenectomy was recommended due to concern for a cyst size greater than 3 cm, apparent growth of the cyst by 3 cm, and the natural history of this presumed IPMN. Access to the abdomen was accomplished and the robot was docked. The procedure was performed using the clockwise technique. The left colon was mobilized from lateral to medial, and the splenic flexure was taken down, followed by the gastrocolic ligament and short gastric vessels, allowing for a full exposure of the pancreas. An ultrasound (US) probe was used to assess the cyst margins. Careful dissection was performed due to extensive scar tissue from the patient’s prior nephrectomy. A distal pancreatectomy and splenectomy was performed uneventfully dividing the pancreas with a linear stapler with reinforcement using the squeeze technique and no drain was left based on current evidence. The patient had no post-operative complications and was discharged on post-operative day five. Pathology was consistent with a 5.5 cm dermoid cyst of the pancreas, negative for malignancy in surrounding lymph nodes, omentum, and spleen (Figure 2 and Figure 3).
The patient was seen in clinic after hospital discharge with an uneventful post-operative course.
Methods
Literature review was conducted using PubMed, Ovid, Clinical Key, Cochrane Library, Web of Science, and UpToDate. Results were not limited by publication date. We reviewed the resulting literature to determine the incidence and prior reported cases, pathology, presentation, imaging findings, and management to previous literature. Reviewed literature included retrospective reviews, review series of pancreatic cysts, radiologic reviews, literature review articles, multiple case series, and multiple case reports.
DISCUSSION
Cystic pancreatic lesions are categorized into one of three main types: true cysts, pseudocysts, and cystic neoplasms [2]. The most common cystic neoplasms are serous cystic neoplasms, mucinous cystic neoplasms, and intraductal papillary mucinous neoplasms which account for about 90% of all cystic pancreatic lesions. True cysts are distinguished by their squamous epithelial lining and benign nature and include lymphoepithelial cysts, epidermoid cysts, and dermoid cysts [2],[3],[4]. Dermoid cysts account for about 0.5% of all pancreatic cysts [5], with the first pancreatic cystic teratoma, or dermoid cyst, reported by Kerr in 1918 [1].
Dermoid cysts are further defined as congenital tumors that can be mature and benign, or, rarely, immature and malignant [3],[5]. Mature dermoid cysts comprise the three embryonic germ layers: ectoderm, endoderm, and mesoderm, and are believed to originate during the closure of the neural groove around week four of development [3],[5]. These tumors are more commonly found in the ovaries and testes but can be found at any site along the route of ectodermal cell migration. This occurs usually in the midline, such as the brain, cranium, mediastinum, omentum, retroperitoneum, and sacrococcygeal regions [6].
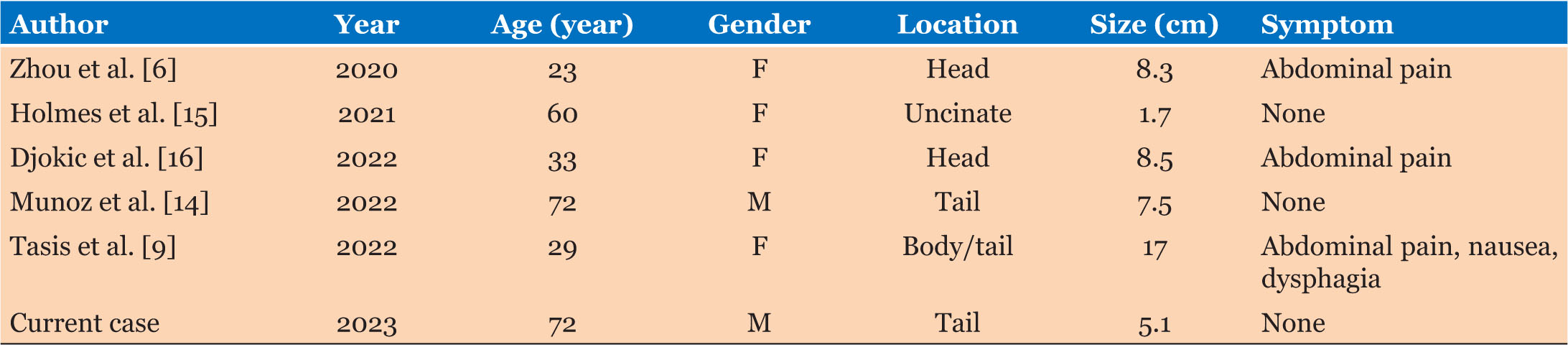
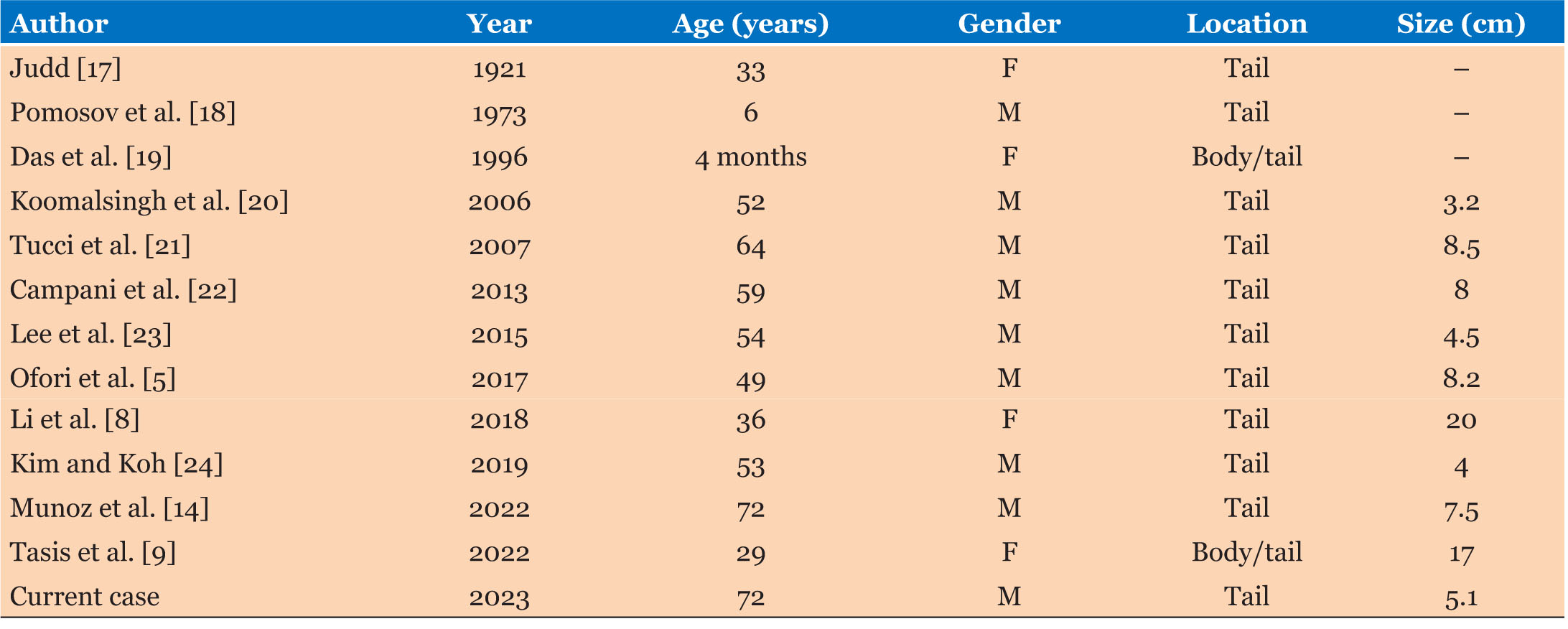
Rarely, dermoid cysts can be seen in the pancreas. Kerr first described this in 1918. Scheele et al. reported a total of 26 published cases of pancreatic dermoid cyst between 1918 and 2010 [7]. By 2020, Li et al. and Zhou et al. separately reported a total of 50 published cases in the literature, of which 54% were male and 46% were female [6],[8]. Our query found an additional five published cases (Table 1) and including our present case, totals 56 published cases of pancreatic dermoid cyst in the literature.
Some literature suggests a slight male predominance [3],[7]; however, others state no gender difference [2],[5]. There is a diverse age range from four months to 74 years [3],[5],[7]. Overall, teratomas are more common among pediatric patients [9]. Of pancreatic dermoid cysts, the majority occur in the body of the pancreas. Li et al. suggested that the most common sites of presentation are pancreatic body (35.6%), the head (33.3%), the tail (15.5%), the body/tail (8.9%), and finally the head/body (6.7%) [8]. Of the 13 published cases of pancreatic dermoid cyst to be located in the pancreas tail, 70% were male (Table 2).
Most pancreatic cysts are asymptomatic and only present as an incidental finding on imaging or postmortem during autopsy [5],[10]. Our case was initially discovered as an incidental finding. If symptomatic, complaints are typically nonspecific gastrointestinal symptoms secondary to size and location of the mass [2],[5],[7],[10]. Li et al. found nonspecific gastrointestinal symptoms, such as abdominal pain, nausea, vomiting, anorexia, abdominal distension, back pain, and weight loss in their query of published cases. These tumors can also present as a palpable abdominal mass when larger in size [4].
Magnetic resonance imaging (MRI) is the current preferred imaging modality for classification [5],[11]. Other imaging modalities used are CT scan and ultrasound (US). Based on the origin of the dermoid cyst, imaging will vary depending on the actual type and proportion of tissue within the cyst, leaving uncertainty when it comes to diagnosis. Typically, more adipose tissue, fat-fluid levels, and calcium within the cyst can be more suggestive of a dermoid cyst [6]. These findings are better detected by CT scan than US. If US is used, the fat within the cyst appears hyperechoic and calcified areas are seen as areas of high intensity with acoustic shadowing [5].
Recently, fine needle aspiration (FNA) guided by endoscopic ultrasound (EUS) has been shown to be a safe and cost-effective technique to detect pancreatic masses. This includes solid tumors, cystic neoplasm, pseudocyst, and reactive changes. Fine needle aspiration seems to be a promising tool to lead to definite preoperative diagnosis and may avoid unnecessary surgery for benign neoplasm [8]. Unfortunately, definitive diagnosis is usually made post-operatively via pathology and cytology results. Microscopically the cyst wall is lined by a single layer of keratinized stratified squamous epithelium with underlying connective tissue. In some instances, gross structures from ectodermal, endodermal, and mesodermal germ layers such as cartilage, bone, hair, teeth, sweat glands, thyroid tissue, etc. can be identified. Tumor markers can be elevated but as these lesions are benign, tumor markers are not typically found to be elevated and, therefore, are not diagnostic [5]. In our case, the patient demonstrated an elevated CA 19-9.
Preoperative diagnosis of dermoid cysts remains difficult because of a combination of their extremely rare nature, vague presentation of symptoms, and sometimes ambiguous imaging findings. Differential diagnosis to consider when dealing with dermoid cysts include pancreatic pseudocyst, serous cystadenoma, mucinous cystadenoma, solid pseudo-papillary tumor, intraductal papillary mucinous neoplasm (IPMN), and epidermoid cyst in the intrapancreatic accessory spleen [6]. Lane et al. differentiate between possible pancreatic lesion imaging and pathology when considering dermoid cyst as a diagnosis [3]. Computed tomography scan findings can hint toward different diagnoses of pancreatic lesions; pancreatic pseudocysts appear as a unilocular cyst in a patient with a history of pancreatitis, serous cystadenoma’s have a fibrous central scar with or without a characteristic stellate pattern of calcification, mucinous cystadenomas have peripheral “eggshell” calcification, solid pseudopapillary tumors have a solid tumor associated with a cystic component, unilocular or multilocular, IPMN’s are septated cysts that communicate with the main pancreatic duct, and dermoid cysts are rounded, well-circumscribed, extremely hypodense lesion with a Hounsfield unit measurement of −20 to −140 [3]. A dermoid cyst can also be differentiated on pathologic findings as a combination of both cystic and solid components, including teeth, hair, cartilage, and dermal appendages such as hair follicles, sweat glands, and abundant sebaceous material [3].
While the malignant degeneration of dermoid cysts in the pancreas has never been reported, it is estimated that 7–10% of other retroperitoneal teratomas are malignant [5]. Rathor et al. and Qin et al. reported in two large case series of mature cystic teratomas of the ovary with overall incidence of malignant transformation ranging from 2.4% to 3.5% [12],[13]. So, while malignant transformation is rare, it is a reported complication of mature cystic teratoma of other organs. Furthermore, of all excised pancreatic cystic lesions, 70% have been reported as malignant or premalignant [5]. Due to this, surgical resection of these lesions is important.
Surgical excision is the principal method for the management of pancreatic dermoid cyst. The type of procedure is highly dependent on the location and size of the tumor [6]. In the absence of malignancy, as in these benign lesions, simple resection is deemed curative with a good prognosis [4],[7]. Procedures such as pancreaticoduodenectomy and distal pancreatectomy remain the current standard of care while procedures such as Roux-en-Y choledochojejunostomy, external cyst drainage, or cyst gastric anastomosis are feasible options as well [6],[14]. These options may resolve symptoms but have been shown to have high recurrence rates. Overall, pancreaticoduodenectomy or distal pancreatectomy decreases the small percentage of malignancy transformation and excision assists in obtaining final diagnosis. In our case, due to the location of the tumor, growth of the tumor, elevated CA 19-9, and uncertain diagnosis we elected for robotic-assisted distal pancreatectomy with splenectomy.
CONCLUSION
We present the case of a 72-year-old male originally referred to us for incidental findings of a pancreatic tail cyst which grew during surveillance imaging. The patient underwent robotic-assisted distal pancreatectomy with splenectomy and subsequent pathology was consistent with 5.5 cm dermoid cyst of the pancreas. Overall, pancreatic dermoid cysts are an extremely rare phenomenon with only 56 reported cases in the literature since 1918. Furthermore, of those reported cases, only a small percentage are located in the pancreatic tail. Preoperative diagnosis of this type of tumor remains extremely difficult because of a combination of their extremely rare nature, vague presentation of symptoms, and sometimes ambiguous imaging findings. Our case presents the first detailed report of a robotic distal pancreatectomy with splenectomy for this pathology. Robotic distal pancreatectomy with splenectomy is safe and feasible in patients with cystic lesions and prior history of ipsilateral retroperitoneal surgery. Further research should focus on pre-operative workup for proper diagnosis and further identification of incidence and prevalence.
REFERENCE
1.
2.
Kubo T, Takeshita T, Shimono T, Hashimoto S, Miki Y. Squamous-lined cyst of the pancreas: Radiological-pathological correlation. Clin Radiol 2014;69(8):880–6. [CrossRef]
[Pubmed]

3.
Lane J, Vance A, Finelli D, Williams G, Ravichandran P. Dermoid cyst of the pancreas: A case report with literature review. J Radiol Case Rep 2012;6(12):17–25. [CrossRef]
[Pubmed]

4.
Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms of the pancreas revisited. Part IV: Rare cystic neoplasms. Surg Oncol 2012;21(3):153–63. [CrossRef]
[Pubmed]

5.
Ofori E, Ramai D, Etienne D, Reddy M, Shahzad G. Large dermoid cyst presenting as recurrent pancreatitis. Gastroenterology Res 2017;10(5):322–4. [CrossRef]
[Pubmed]

6.
Zhou XH, Ma JK, Valluru B, Sharma K, Liu L, Hu JB. Diagnosis and differentiation of mature cystic teratoma of pancreas from its mimics: A case report. Medicine (Baltimore) 2020;99(47):e23267. [CrossRef]
[Pubmed]

7.
Scheele J, Barth TFE, Wittau M, Juchems M, Henne-Bruns D, Kornmann M. Cystic teratoma of the pancreas. Anticancer Res 2012;32(3):1075–80.
[Pubmed]

8.
Li Z, Ke N, Liu X, Gong S. Mature cystic teratoma of the pancreas with 30 years of clinical course: A case report. Medicine (Baltimore) 2018;97(15):e0405. [CrossRef]
[Pubmed]

9.
Tasis N, Prountzopoulou AA, Skafida E, et al. Giant teratoma of the pancreas expanding to the mediastinum: Rare tumor and literature review. Rare Tumors 2022;14:20363613221147470. [CrossRef]
[Pubmed]

10.
Kim YS, Cho JH. Rare nonneoplastic cysts of pancreas. Clin Endosc 2015;48(1):31–8. [CrossRef]
[Pubmed]

11.
D’Haese JG, Werner J. Surgery of cystic tumors of the pancreas – Why, when, and how? Visc Med 2018;34(3):206–10. [CrossRef]
[Pubmed]

12.
Rathore R, Sharma S, Agarwal S. Malignant transformation in mature cystic teratoma of the ovary: A retrospective study of eight cases and review of literature. Prz Menopauzalny 2018;17(2):63–8. [CrossRef]
[Pubmed]

13.
Qin L, Zhao T, Liu X, et al. Malignant transformation arising from mature ovarian cystic teratoma: A case series. Medicine (Baltimore) 2021;100(13):e24726. [CrossRef]
[Pubmed]

14.
Munoz C, Lindner C, Pizarro F, Pino C. Mature cystic teratoma of the pancreas: An unusual indication for laparoscopic distal pancreatectomy. Arq Bras Cir Dig 2022;35:e1693. [CrossRef]
[Pubmed]

15.
Holmes I, Ha NB, Peng Y, Kaltenbach T. A dermoid cyst presenting as a hypoechoic pancreatic mass. Am J Gastroenterol 2021;116(3):449. [CrossRef]
[Pubmed]

16.
Djokic M, Hadzialjevic B, Rankovic B, Dezman R, Tomazic A. Neuroendocrine tumor arising within mature cystic teratoma of the pancreas: literature review and case report. Curr Oncol 2022;29(7):4717–24. [CrossRef]
[Pubmed]

18.
Pomosov DV, Shpit’ko FS, Volianiuk MA. Dermoid cyst of the pancreas in a child. [Article in Russian]. Vestn Khir Im I I Grek 1973;110(4):92–3.
[Pubmed]

19.
Das PC, Radhakrishna K, Rao PL. Cystic teratoma of the pancreas. Pediatr Surg Int 1996;11(2–3):177–8. [CrossRef]
[Pubmed]

20.
Koomalsingh KJ, Fazylov R, Chorost MI, Horovitz J. Cystic teratoma of the pancreas: Presentation, evaluation and management. JOP 2006;7(6):643–6.
[Pubmed]

21.
Tucci G, Muzi MG, Nigro C, et al. Dermoid cyst of the pancreas: Presentation and management. World J Surg Oncol 2007;5:85. [CrossRef]
[Pubmed]

22.
Campani D, Gallippi G, Granai M, De Lio N, Boggi U. Dermoid cyst of the pancreas. Int J Colorectal Dis 2013;28(4):597–8. [CrossRef]
[Pubmed]

23.
Lee SE, Choi YS, Hong SU, Oh HC, Lee ES. Dermoid cyst of the pancreas: A rare cystic neoplasm. Int J Surg Case Rep 2015;17:72–4. [CrossRef]
[Pubmed]

24.
Kim H, Koh YS. Mature cystic teratoma of the pancreas: A rare cystic neoplasm. Open Med (Wars) 2019;14:872–4. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
William Cobb - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jessica Marks - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Tien-Anh Tran - Analysis of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Armando Rosales - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2025 William Cobb et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.