|
Case Report
Infected hemorrhagic pancreatic pseudocyst: A rare complication of acute pancreatitis
1 General Practitioner, Department of Internal Medicine, Siloam Hospitals Kebon Jeruk, Jakarta, Indonesia
2 Internist, Department of Internal Medicine, Siloam Hospitals Kebon Jeruk, Jakarta, Indonesia
3 Digestive Surgeon, Department of Surgery, Siloam Hospitals Kebon Jeruk, Jakarta, Indonesia
Address correspondence to:
Dian Daniella
Jalan Bisma 6 blok B 12 nomor 17, Jakarta Utara, DKI Jakarta,
Indonesia
Message to Corresponding Author
Article ID: 100009G01DD2019
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Daniella D, Wiguna C, Jeo WS. Infected hemorrhagic pancreatic pseudocyst: A rare complication of acute pancreatitis. Edorium J Gastroenterol 2019;6:100009G01DD2019.ABSTRACT
Introduction: Pancreatitis is an uncommon disease defined as an inflammatory disorder of the pancreas that can be acute or chronic. After four weeks, unresolved acute pancreatitis can result in pancreatic pseudocyst. Usually pancreatic pseudocyst formed in the absence of pancreatic necrosis, although rare but exception has been reported and it affects the treatment of choice. Pseudocyst must be managed correctly due to its high mortality complication, such as bleeding pseudoaneurysm.
Case Report: A 51-year-old man presented to the emergency room with his third episode of abdominal pain. In one month prior, the patient had already been hospitalized twice with acute pancreatitis. He came with severe abdominal pain three hours prior to admission. Patient had a history of alcohol abuse. Physical examination revealed tachycardia, afebrile, distended, and rigid abdomen with no bowel sound. Laboratory examination revealed high leucocyte (35,200/uL), high amylase (1063 U/L), and high lipase (540 U/L). Abdominal computed tomography (CT) scan revealed enlargement of pancreas body and tail with heterogenous density (necrotic), with around 11 cm mass containing fluid mean 70 HU (blood) in pancreas body. One week before the abdominal CT scan showed acute pancreatitis with no cyst. The patient was kept fasting with parenteral nutrition, antibiotic, octreotide subcutaneously, opioid, and laparoscopic drainage and debridement was scheduled. During surgery, mass containing approximately 1000 mL of fluid and blood clot was seen and drained. The patient did well after the surgery.
Conclusion: Diagnosis and prompt treatment of pseudocyst is important. In a patient with pseudocyst and necrotizing pancreatitis, surgical drainage is the treatment of choice.
Keywords: Hemorrhagic, Laparoscopic drainage, Pancreatitis, Pseudocyst
INTRODUCTION
Pancreatitis is an uncommon disease defined as an inflammatory disorder of the pancreas that can be acute or chronic [1]. The annual incidence of acute pancreatitis ranges from 13 to 45 per 100,000 persons worldwide with mortality around 1–7% which increases to around 10–18% in severe pancreatitis [2]. After four weeks, unresolved acute pancreatitis can result in pancreatic pseudocyst. Pancreatic pseudocysts are collection of pancreatic fluid surrounded by a non-epithelial perimeter in the peripancreatic tissues and a rare complication in acute pancreatitis [3],[4]. Usually pancreatic pseudocyst formed in the absence of pancreatic necrosis, although rare but exception has been reported and it affects the treatment of choice [2]. Pseudocyst must be managed correctly due to its complication, such as infected pseudocyst, rupture, thrombosis, mass effect, and bleeding pseudoaneurysm [3]. Mortality rate of bleeding pseudoanuerysm is very high (40%) [5], therefore early diagnosis and prompt treatment of pancreatic pseudocyst is important. This case report presents a male with a rare infected hemorrhagic pseudocyst with necrotizing pancreatitis and its choice of treatment.
CASE REPORT
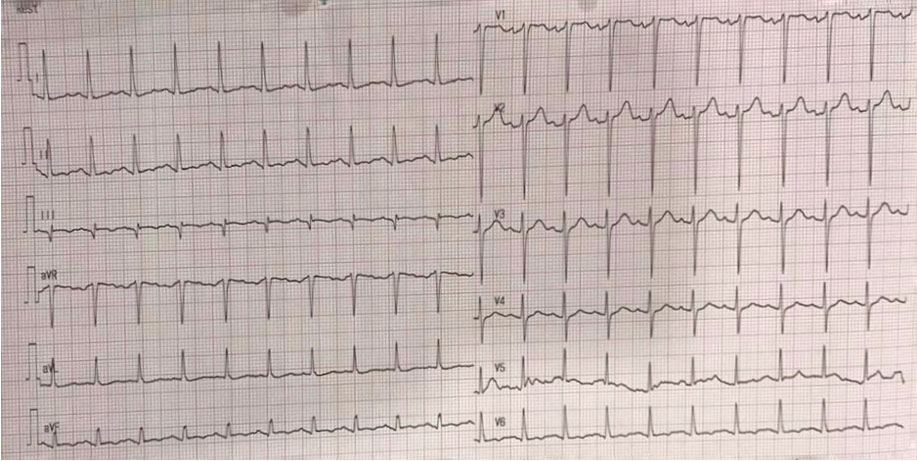
A 51-year-old man presented to the emergency room with his third episode of abdominal pain. The patient had already been hospitalized twice with acute pancreatitis in the last month. He came with severe abdominal pain three hours prior to admission. The patient had a history of alcohol abuse and had already undergone cholecystectomy. He had hypertension. Physical examination revealed tachycardia, afebrile, distended, and rigid abdomen with no bowel sound (Figure 1).
Laboratory results revealed hemoglobin (Hb) 17.2 g/dL (13.0–18.0), hematocrit 47.9% (40–54), white blood cell (WBC) 35,200/uL (4000–10,000), platelets 553,000/uL (150,000–400,000), pancreatic amylase 1063 U/L (<53), lipase 540 U/L (8–78), aspartate aminotransferase (AST) 48 mg/dL (5–34), alanine aminotransferase (ALT) 98 mg/dL (<55), creatinine 1.0 mg/dL (0.7–1.3), natrium 136 mmol/L (134–145), kalium 4.2 mmol/L (3.5–5.5), chloride 99 mmol/L (97–111), calcium 9.2 mg/dL (8.4–10.2), total bilirubin 2.48 mg/dL (0.2–1.2), conjugated bilirubin 1.69 mg/dL (0–0.5), unconjugated bilirubin 0.79 mg/dL (0–0.7), and blood glucose 122 mg/dL.
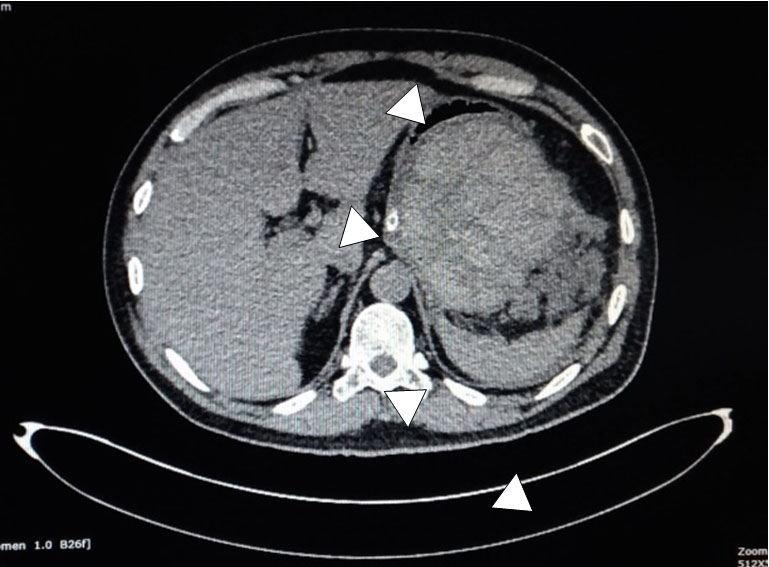
Abdominal CT scan revealed normal biliary duct, enlargement of pancreas body and tail with heterogenous density (necrotic), with around 11 cm mass containing fluid mean 70 HU (blood) in pancreas body, pushing gaster to right anterolateral, fluid accumulation with heterogenous density (mean HU 0–20) in left paracolic gutter and perilienalis (necrotizing collection), ascites in perilienalis and left–right paracolic gutter, fat stranding peripancreatic, with bilateral pleural effusion (Figure 2). One week before the abdominal CT scan showed acute pancreatitis with no mass. Magnetic resonance cholangiopancreatography (MRCP) from two weeks prior showed normal ducts.
Patient was kept fasting with parenteral nutrition, antibiotic intravenous (piperazilin 4 gram/tazobactam 0.5 gram three times daily), octreotide subcutaneously, and opioid (pethidine). Laboratory examination was repeated the next day and it showed increasing WBC from 35,200/uL to 45,800/uL.
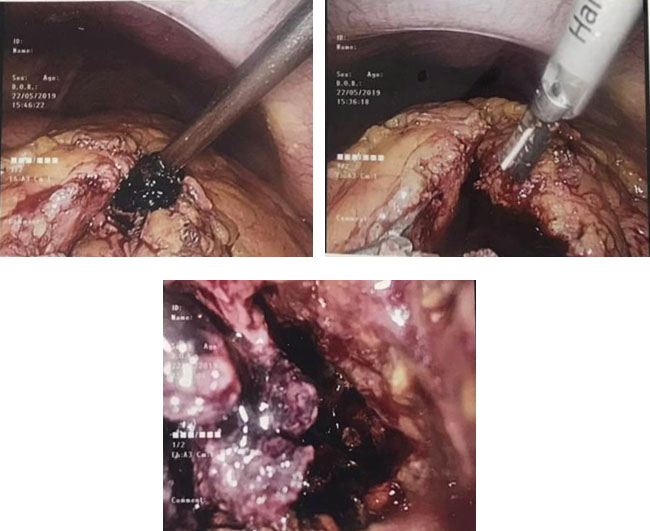
For this patient, laparoscopic drainage was considered. During surgery, mass containing approximately 1000 mL of fluid and blood clot was seen and drained. The patient did well after surgery (Figure 3).
After surgery, his symptoms decreased and remained afebrile. In postoperative day 3, laboratory results revealed WBC 16,000/uL (4000–10,000), platelets 553,000/uL (150,000–400,000), pancreatic amylase 48 U/L (<53), lipase 42 U/L (8–78). The patient continued to show good clinical improvement in hospitalization. He started having normal meals and was discharged from the hospital in good clinical status.
DISCUSSION
Definition
Pancreatic pseudocyst is collection of pancreatic fluid surrounded by a non-epithelial perimeter in the peripancreatic tissues and a rare complication in acute pancreatitis [3],[4].
Etiology and pathogenesis
Pancreatic pseudocyst is more common after alcohol-induced than after non-alcohol-related pancreatitis. Other causes of pancreatic pseudocyst are biliary tract disease, trauma to the pancreas or idiopathic [6]. Pancreatic pseudocyst is often associated with a non-necrotizing pancreatitis and arises from disruption of the main pancreatic duct, resulting in leakage of pancreatic enzyme to retroperitoneum or peripancreatic tissue [3]. Subsequent leakage of pancreatic juice leads to a localized accumulation of clear fluid, forming a collection, usually after four weeks [6]. Although rare, pancreatic pseudocyst may also rise from acute necrotizing pancreatitis when the necrotic areas of the neck or body of the pancreas is isolated with a still-functional distal pancreatic part [3]. In chronic pancreatitis, acute fluid exacerbation, blockage of the main pancreatic duct from a protein plug or calculus can lead to the pseudocyst formation [7]. This patient already has acute pancreatitis approximately four weeks and in third hospitalization it showed hemorrhagic pancreatic pseudocyst with necrotizing pancreatitis.
Signs and symptoms
The patient came due to severe abdominal pain and dyspepsia. Although usually asymptomatic, other symptoms that may arise from the patient with pseudocyst are pain, vomiting, nausea, jaundice, and weight loss [4],[7]. Symptoms related to pseudocyst arise from local mass effect or infection of the pseudocyst [3].
The sensitivity of physical examination findings is limited. Patients frequently have a tender abdomen and occasionally have a palpable abdominal mass. Peritoneal signs suggest rupture of the cyst or infection. Other possible findings include fever, scleral icterus, or pleural effusion [6].
Laboratory evaluation
Amylase and lipase levels are often elevated, but may be within reference ranges. Other laboratory values, such as serum bilirubin and ALT/AST, may be elevated if the bile duct is obstructed from stone, extrinsic compression from the pseudocyst, or from underlying liver disorder. Laboratory values have limited values in diagnostic pancreatic pseudocyst [6].
Imaging modalities
Multiple imaging modalities can be used to diagnose pancreatic pseudocyst. Transabdominal ultrasound (US) is most frequently used for tools, but it is operator dependent and has imaging limitations, such as overlying bowel gas. Its sensitivity ranges from 70% to 90% [7]. Pancreatic pseudocyst appears as an echoic structure associated with distal acoustic enhancement on US examination. They are well defined and round or oval, and they are contained within a smooth wall. During the early phases of their development, pseudocysts can appear more complex, with varying degrees of internal echoes, results from the presence of necrotic debris and are more common in pseudocysts that form as a result of acute necrotizing pancreatitis than in chronic pancreatitis-related pseudocysts [6].
Abdominal CT scan is useful in differentiating pseudocyst and walled-off pancreatic necrosis due to its ability to differentiate solid and debris. Its sensitivity ranges from 90% to 100% [7]. Pancreatic pseudocyst will appear as thick-walled, rounded, fluid-filled mass adjacent to the pancreas. Positive CT findings in patient with history of pancreatitis do not require confirmation with another imaging modality. In the acute setting, CT scan is the better choice because significant amounts of bowel gas resulting from ileus or obstruction decrease the sensitivity of US. In addition, CT scans provide more detailed information regarding the surrounding anatomy. The major weakness of CT scan is the relative inability to differentiate pseudocyst from and cystic neoplasm of pancreas [6].
In order to differentiate pseudocyst from and cystic neoplasm of pancreas, fine needle aspiration (FNA) is done. This can be done with endoscopic ultrasound (EUS) guided [6].
Next imaging modalities are magnetic resonance imaging (MRI) and MRCP, where they are the most accurate and sensitive diagnostic tool to evaluate pancreatic duct. These modalities are useful to exclude pancreatic duct disruption [7]. They are not routinely used but MRI is superior to CT in depicting debris within fluid collections and pseudocysts [6].
Complication
Complication of pancreatic pseudocyst can happen in acute and chronic setting. Acute complications include bleeding (usually from splenic artery pseudoaneurysm), infection, and rupture. Mortality rates due to bleeding pseudoaneurysms can be up to 40% due to unpredictable timing of rupture. Pseudoaneurysm often happen with hemorrhagic pseudocysts due to established pseudocyst eroding into a visceral artery, thereby converting the pseudocyst into a large pseudoaneurysm or a pseudocyst eroding the bowel wall with bleeding from the mucosal surface. Therefore in presence of hemorrhagic pseudocyst, possibility of pseudoaneurysm should be considered [5]. It typically presents as abdominal pain and bleeding in gastrointestinal tract or peritoneal cavity or simultaneously into more than one of these sites [6],[7]. In this patient, pseudoaneurysm was not found.
Infection occurs either spontaneously or after therapeutic or diagnostic manipulations. While infected pseudocyst can initially be treated with conservatively, majority of patients will require intervention. Rupture into the gastrointestinal tract or peritoneal cavity usually requires emergent surgical exploration [6].
Chronic complications include gastric outlet obstruction, biliary obstruction, splenic complication, and portal hypertension. Biliary obstruction occurs due to a large cyst in the pancreatic head region obstructing the common bile duct and resulting in obstructive jaundice. Therapeutic endoscopy with short-term biliary stenting is valuable in this situation. It can be retained until either the pseudocyst resolves or is treated by intervention [6].
Splenic complications of pseudocyst include massive hemorrhage into the pseudocyst, sepsis with splenic infarction, and splenic vein thrombosis. The diagnosis of intrasplenic pseudocyst should be suggested by the presence of a mass in the left upper quadrant. Selective celiac arteriography should be performed whenever splenic involvement is suggested in order to confirm the diagnosis and to search for pseudoaneurysm formation with resection of the pseudocyst by splenectomy and distal pancreatectomy is the treatment of choice [6].
Portal hypertension can result from compression or obstruction of the splenic vein/portal vein either by the cyst alone or in conjunction with underlying chronic pancreatitis. Appropriate surgical procedure can effectively treat this form of portal hypertension [6].
Management
Intravenous fluids, analgesics, and antiemetics are routinely given. For patients who can tolerate oral intake and low fat diet are recommended. In patients who cannot tolerate oral nutrition, support can be provided via nasoenteral feeding or total parenteral nutrition (TPN). Jejunal feeding will be related to fewer complications but has lower calories than TPN. The rationale of using octreotide as a therapy for pancreatic pseudocyst is that it will decrease pancreatic secretions and aid in pseudocyst resolution [6].
Routine antibiotic proylactic is not associated with significant decrease in mortality and morbidity, but in the presence of infection, antibiotic should be given [8]. The timing of infection in acute pancreatitis may vary, but usually happens between second and fourth weeks after the onset of pancreatitis. In the presence of clinical parameters, such ac high WBC, high C-reactive protein (CRP), and high procalcitonin; pancreatic necrosis in imaging; or sample of infected area shows infection, antibiotic should be initiated [9]. Antibiotic can be used as third-generation cephalosporin, piperazilin/tazobactam, quinolone and carbapenem, and metronidazole. Antibiotic can be given for 7–10 days. There is no or minimal residual infection after a source control infection [8].
Pseudocyst can be managed conservatively or with intervention [3]. It can resolve spontaneously [4], but pseudocysts larger than 6 cm or those that were enlarging and symptomatic require intervention [10]. Main indications for pancreatic pseudocyst intervention are rupture, pain, biliary obstruction, gastric or duodenal obstruction, increasing size on follow-up, bleeding pseudocyst, and infected pseudocyst [7].
Interventions for pseudocyst are percutaneous drainage (PD), endoscopic drainage (ED), and surgical drainage (SD). Percutaneous drainage is useful for patients with severe morbidities that cannot tolerate SD or ED. With US guidance, a drainage pigtail catheter is placed percutaneously into the fluid cavity and fluid is drained. The fluid is collected over several weeks into an external collection system. When the drainage output becomes minimal, the catheter is removed. Limitation for PD is scarcity of access route [7], external drain tends to create significant discomfort and may require repositioning or exchange, and cannot be done in patients with main pancreatic duct stricture and bloody or solid material cyst [6].
Endoscopic drainage can be performed either via transpapillary drainage (TPD) or transmural (TSM), with or without the use of EUS. Transpapillary drainage can be performed whenever there is a communication between the pseudocyst and the pancreatic duct, which can be demonstrated with endoscopic retrograde cholangiopancreatography (ERCP) and is also indicated in cysts that are too distant (>1 cm) from the gastrointestinal lumen to allow safe TSM drainage. With this technique a stent can be passed through the pancreatic duct into the pseudocyst and if there is a ductal leak or structure, stenting can bridge the leak or dilate the stricture. On the other hand, TSM drainage requires close proximity of the pseudocyst with the gastrointestinal lumen and endoscopic localization in the form of a visible luminal bulge. Endoscopic ultrasound allows the drainage of nonbulging pseudocysts, with a cyst-lumen distance of 1–1.5 cm, under direct visualization, avoiding complications [7].
Surgical drainage can be done by laparotomy or laparoscopically. The advantage of surgical drainage is it can create wider stoma for drainage [7]. If a pseudocyst is accompanied with pancreatic necrosis, SD is preferred because necrotic debris can be retrieved while eliminating the fluid within pseudocyst [11],[12]. Laparoscopic drainage is considered to be a safe and effective method and associated with less postoperative pain, shorter length of stay, and non-inferior success rate compared to laparotomy [4]. In this case, the patient had pancreatic necrosis with large retroperitoneal pseudocyst, therefore surgical drainage was considered for this patient.
CONCLUSION
In conclusion, pancreatic pseudocyst can cause a high mortality complication, such as bleeding pseudoaneurysm. Pseudocyst can be managed conservatively, surgically, and endoscopically. For patients with pseudocyst and necrotizing pancreatitis, surgical drainage is the treatment of choice.
REFERENCE
1.
Srettabunjong S, Limgitisupasin W. Severe acute hemorrhagic pancreatitis secondary to cholelithiasis as a rare cause of sudden unexpected death in medico-legal case: A case report. Medicine (Baltimore) 2016;95(34):e4680. [CrossRef]
[Pubmed]

2.
Evans RP, Mourad MM, Pall G, Fisher SG, Bramhall SR. Pancreatitis: Preventing catastrophic haemorrhage. World J Gastroenterol 2017;23(30):5460–8. [CrossRef]
[Pubmed]

3.
Gurusamy KS, Pallari E, Hawkins N, Pereira SP, Davidson BR. Management strategies for pancreatic pseudocysts. Cochrane Database Syst Rev 2016;4:CD011392. [CrossRef]
[Pubmed]

4.
5.
Ryu K, Hong SS, Cha H, et al. A pancreatic hemorrhagic pseudocyst with pseudoaneurysm and the role of doppler ultrasonography: A case report. Rev Assoc Med Bras (1992) 2019;65(2):123–6. [CrossRef]
[Pubmed]

6.
Habashi S, Draganov PV. Pancreatic pseudocyst. World J Gastroenterol 2009;15(1):38–47. [CrossRef]
[Pubmed]

7.
Agalianos C, Passas I, Sideris I, Davides D, Dervenis C. Review of management options for pancreatic pseudocyst. Transl Gastroenterol Hepatol 2018;3:18. [CrossRef]
[Pubmed]

8.
Verde F, Fishman EK, Johnson PT. Arterial pseudoaneurysms complicating pancreatitis: Literature review. J Comput Assist Tomogr 2015;39(1):7–12. [CrossRef]
[Pubmed]

9.
Hoshimoto S, Aiura K, Shito M, Kakefuda T, Sugiura H. Successful resolution of a hemorrhagic pancreatic pseudocyst ruptured into the stomach complicating obstructive pancreatitis due to pancreatic cancer: A case report. World J Surg Oncol 2016;14(1):46. [CrossRef]
[Pubmed]

10.
Leppäniemi A, Tolonen M, Tarasconi A, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg 2019;14:27. [CrossRef]
[Pubmed]

11.
De Waele JJ. Rational use of antimicrobials in patients with severe acute pancreatitis. Semin Respir Crit Care Med 2011;32(2):174–80. [CrossRef]
[Pubmed]

12.
Braha J, Tenner S. Fluid collections and pseudocysts as a complication of acute pancreatitis. Gastrointest Endosc Clin N Am 2018;28(2):123–30. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Dian Daniella - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Candra Wiguna - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
WIfanto Saditya Jeo - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guarantor of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2019 Dian Daniella et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.