|
|
Original Article
| ||||||
| Endocytic pathway in Caco-2 cells: A model for drugs transport across the intestinal epithelial cell barrier | ||||||
| Mohammed A. Akeel | ||||||
|
Chairman of the Department of Anatomy, Faculty of Medicine, Jazan University, Jazan, Kingdom of Saudi Arabia
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article] [Similar article in PubMed] [Similar article in Google Scholar] |
| How to cite this article |
| Akeel MA. Endocytic pathway in Caco-2 cells: A model for drugs transport across the intestinal epithelial cell barrier. Edorium J Gastroenterol 2018;5:100008G01MA2018. |
|
ABSTRACT
| ||||||
|
Aims: Human colon adenocarcinoma (Caco-2 cells), has the ability to spontaneously differentiate towards a normal intestinal epithelium phenotype in long-term culture. Since the junctional complex, brush border, and transcytosis contribute mainly to the function of enterocytes in absorption, morphological structure of these cells plays undeniable rule. However, full morphological characterization of these cells was not adequately investigated. Therefore, the aim of the present work is to carry full morphological characterization of the cultured intestinal epithelial cells versus the Caco-2 cells model. Methods: Freshly isolated and late cultured jejunal cells of adult rabbits as well as Caco-2 cells were processed for transmission electron microscopy (TEM) and scanning electron microscopy (SEM) and examined with Philips CM100 at 80 KV, and Philips SEM XL 30 at 30 Kv; respectively. Immune-fluorescence examination was done using two primary antibodies: anti-pancytokeratin for detecting cells of epithelial origin and anti-HBB, specific for apical uptake of sucrase-isomaltase, then processed for DAB staining. Immune-electron microscopy was done by tracing the uptake and transport of IgG conjugated to HRP, stained with DAB cytochemistry, and then processed for TEM. Results: Cultured Intestinal epithelial cells expressed early cytokeratin, tight junctional complexes, desmosomes and long microvilli with TEM before deterioration and conversion into fibroblastic like cells on day 21. Caco-2 cells in culture gradually undergo spontaneous enterocytic differentiation, in the form of cytokeratin labeling and positive sucrase-isomaltase as detected by immunofluorescence. Examination by TEM and SEM showed high density surface microvilli, coated pits and vesicles, apical tubo-vesicular system and apical junctional complexes. Immunoelectron microscopy of the Caco-2 cells showed the uptake at the surface of microvilli and transcytosis of IgG-HRP reaching the basal region. Conclusion: This investigation provides full morphological characterization of the cultured epithelial cells and Caco-2 cells model. Caco-2 cells model has proved to be a useful in vitro model that mimics the intestinal epithelium. Keywords: Caco-2 cells, IgG HRP, Scanning electron microscopy, Transmission electron microscopy | ||||||
|
INTRODUCTION
| ||||||
|
Since the first attempt to culture the intestinal epithelial cells [1], it was found that many fibroblastic cells as well as epithelial cells proliferated in primary culture [2]. A key feature of the epithelial cells is the ability to maintain the specific biochemical composition of the apical and basolateral plasma membrane domains while selectively allowing transport of proteins and lipids from one pole to the opposite by transcytosis [3], [4], [5]. Clathrin-mediated endocytosis is the best characterized endocytic mechanism and is the predominant pathway for macromolecule uptake along epithelia [6], [7]. Moreover, receptor-mediated endocytosis is an essential mechanism for the transport of a variety of macromolecules into cells as well as across epithelia [8]. Fc receptors form FcRn that traffics IgG in both directions across polarized epithelial cells that line mucosal surfaces, contributing to host defense [9]. Another characteristic feature of the epithelial cells is the presence of junctional complex at the lateral cell walls, providing barrier against the penetration of noxious substances from the gut lumen [10]. These intercellular junctions associate with the peri-junctional actin cytoskeleton. The human colon adenocarcinoma (Caco-2) cell line is an immortal culture line that spontaneously differentiates in culture to form confluent monolayers that resembles the small intestinal epithelium [11]. Caco-2 cells have been explored regarding its similarity with the intestinal epithelial characteristics [12]. Sucrase-isomaltase is an apical membrane disacharidase that is found exclusively in enterocytes of adult intestine and is expressed in a complex pattern along the intestinal crypt-villus axis [13]. The major site of expression of the enzyme sucrase-isomaltase (SI) is the brush border of the small intestine [14]. Caco-2 cells have been used extensively for analyzing the expression and biosynthesis of the enzyme sucrase-isomaltase (SI) and other brush border hydrolases [15],[16]. The Caco-2 cell line is widely used with in vitro assays to predict the absorption rate of candidate drug compounds across the intestinal epithelial cell barrier. Since the tight junctions of gastrointestinal tract epithelial cells contribute mainly to the physical barrier in absorption, morphological structure of these cells plays undeniable rule. With the widespread use of Caco-2 cell culture as a model for intestinal epithelial absorption, the question of using culture cells derived directly from the intestinal epithelium arises. However, full morphological characterization of these cells was not adequately investigated. Therefore, the aim of the present work is to investigate an in vitro long term cell culture of rabbit intestinal epithelial cells versus the Caco-2 cell line. Full morphological and functional characterization is achieved using transmission and scanning electron microscopy and immunocytochemistry. It aims also at investigating the endocytic pathway at Caco-2 cell line utilizing Goat anti-rabbit IgG conjugated to HRP. | ||||||
|
MATERIALS AND METHODS
| ||||||
|
Isolation and culture of jejunal epithelial cells Under laminar flow hood with strict antiseptic measurements, the mucosa was stripped off the underlying submucosa, and then minced into small pieces. Some of these pieces were transferred directly to culture wells (explants culture). Other pieces (1 mm3) were transferred to 1.8% collagenase and 0.05% elastase (collagenase culture) (Worthington Biochemical, Freehood, NJ). The culture medium was C-199 medium, supplemented with 1% antibiotics-antimycotic and 10% fetal bovine serum (FBS) (GIBCO, Grand Island, NY). The minced mucosa was incubated for 90 min at 37ºC in a shaking water bath. Repeated aspiration of the cell suspension was done every 15 minutes during digestion to insure cell dispersion. The cellular suspension was then filtered and pellets were washed twice in phosphate -buffered solution (pH 7.5) then resuspended in C-199 medium containing 10% FBS and 1% antibiotics -antimycotics and platted onto 25 cm2 cell culture flasks (Fisher, Springfield, NJ). They were incubated in humidified atmosphere of 95% air, 5% CO2 at 37ºC for a period of two weaks. Media were changed every other day. The cells were isolated from the floor of the culture flasks (Falcon, Heidelberg, Germany) by trypsinization. The jejunal epithelial cells were examined by the phase contrast microscope throughout all the culture periods, and the morphological structure of the crypts and cells was described.
Caco-2 cells culture Caco-2 cells were maintained at low density seeding (120–103 cells/cm2) and subcultured every six days (passage frequency). The culture medium used was Dulbecco’s modified Eagle’s minimum essential medium (DMEM) containing 25 mM glucose (Gibco Grand Island, N Y) supplemented with 20% heat-inactivated (56°C, 30 minutes) fetal bovine serum and 1% nonessential amino acids (Gibco, Glasgow, Scotland), and cultured in a 5% CO2/ 95% air atmosphere. The medium was changed every other day. Phase Contrast microscopy was used to examine the morphology of the Caco-2 cell culture throughout the various periods of culture.
Transmission electron microscopy (TEM)
Scanning electron microscopy (SEM)
Immunofluorescence 1- 1:200 monoclonal anti-pancytokeratin (C1801-Sigma). 2- 1:100 Monoclonal antibody anti-Hemoglobin, Beta (HBB 2/614188) (from ascites fluid) specific for sucrase-isomaltase from normal human small intestine was used (Bio Center of the University of Basel, Basel, Switzerland). Indirect Immunofluorescence was performed on jejunal cells monolayers three days old culture and Caco-2 cells (30–40 passages) at least one month old cultures that were seeded into single well culture chamber slides (Fisher, Springfield, and N.J). The cells were fixed for 15 min at room temperature in 3.7% paraformaldehyde in Ca 2+ and Mg2+ free phosphate buffered saline. Cells were initially incubated with blocking serum (5% goat serum 1% BSA) after washing in several changes of PBS. The culture chambers were incubated with the primary antibodies; anti–Pancytokeratin and anti-HBB in PBS, supplemented with 1% BSA for 60 minutes in a humidified 5% CO2 incubator (37°C). The secondary antibody (anti-mouse IgG FITC conjugate) was applied at 1: 128 dilution and the cells were subsequently in cubated at 37°C for 45 minutes. The cells were then washed three times in PBS at room temperature for 10 min each. The chambers were drained and disassembled and slides were mounted with Flouromount G (Fisher) and sealed with a cover slip and examined under an epifluorescence microscope equipped with a blue filter. As controls, several culture chambers were incubated with PBS and secondary (no primary) to detect non-specific binding of secondary antibody. Moreover; the cells were viewed in the absence of either primary or secondary antibodies to identify any auto-fluorescence.
Immunoelectron microscopy
| ||||||
|
RESULTS
| ||||||
|
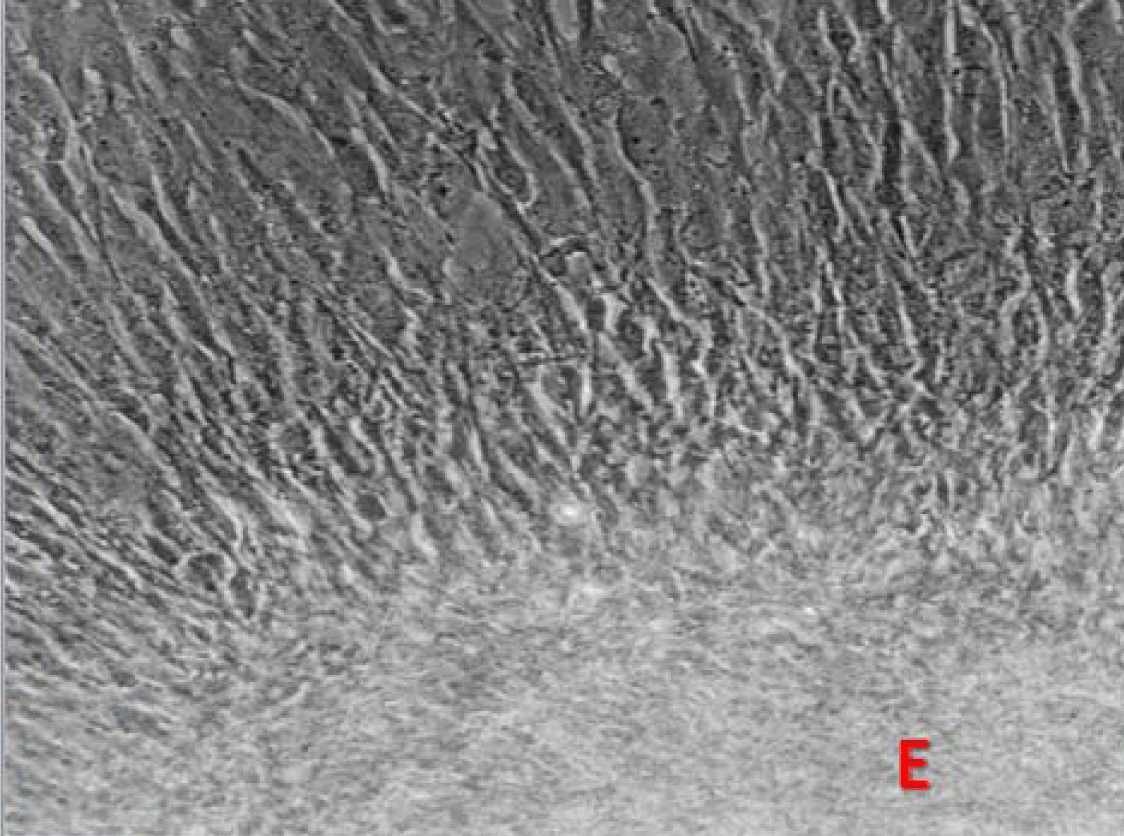
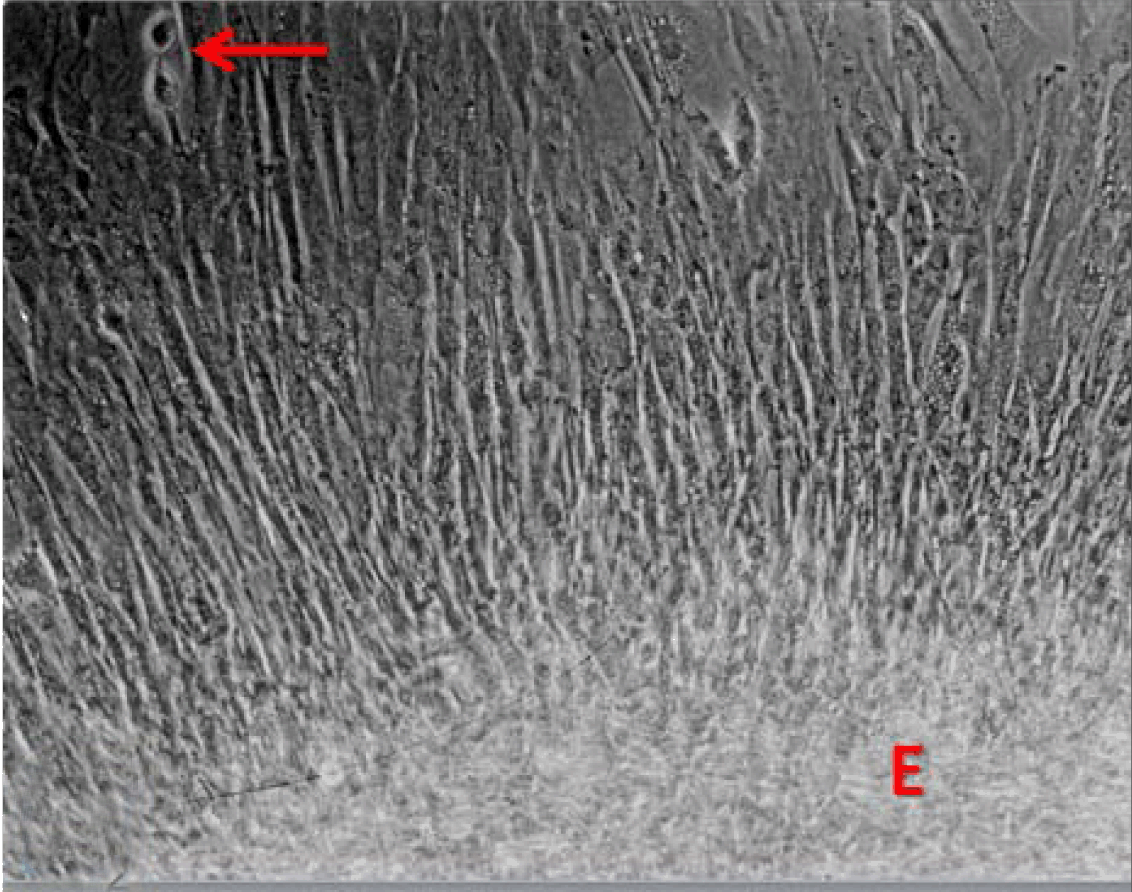
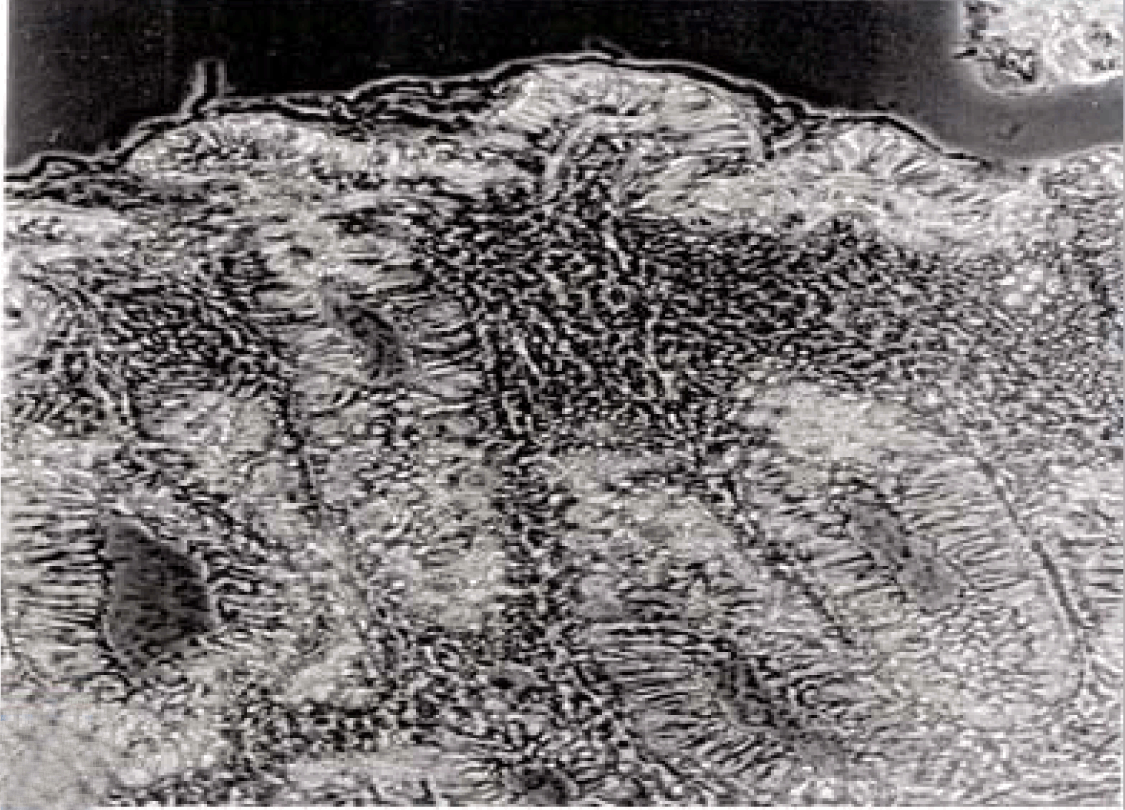
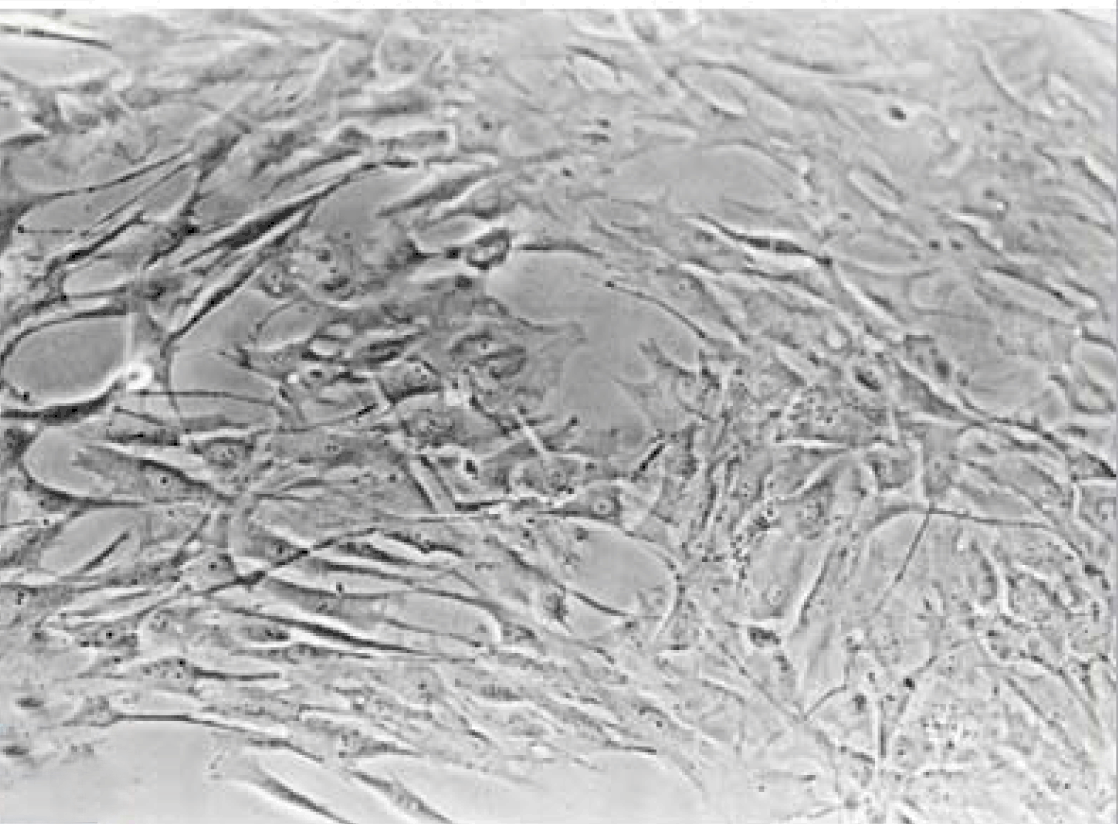
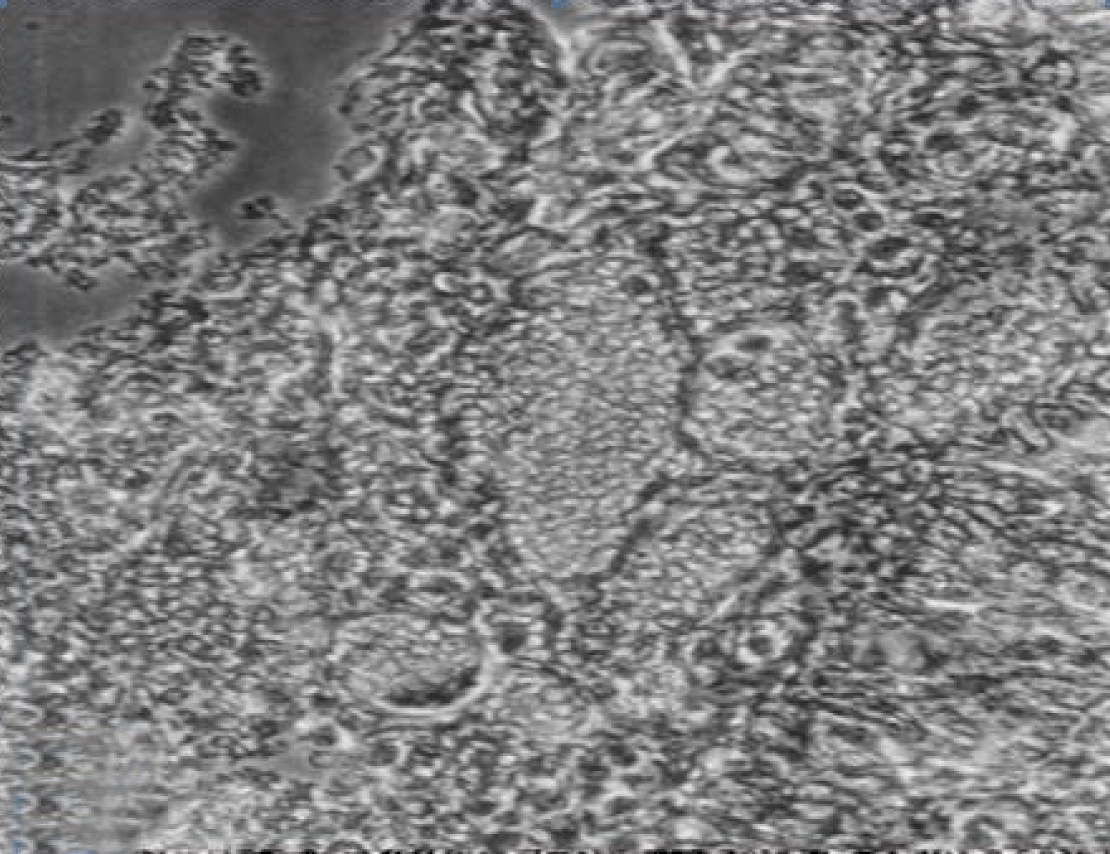
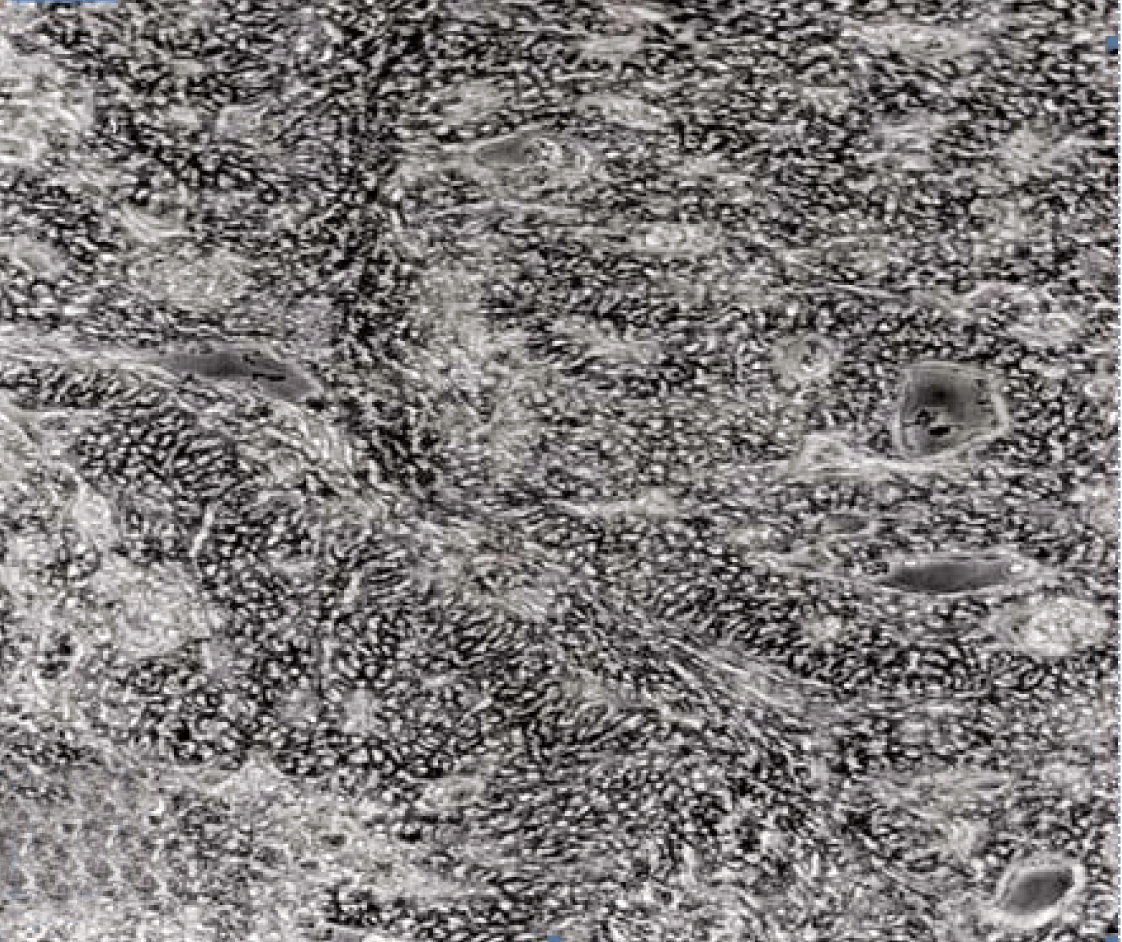
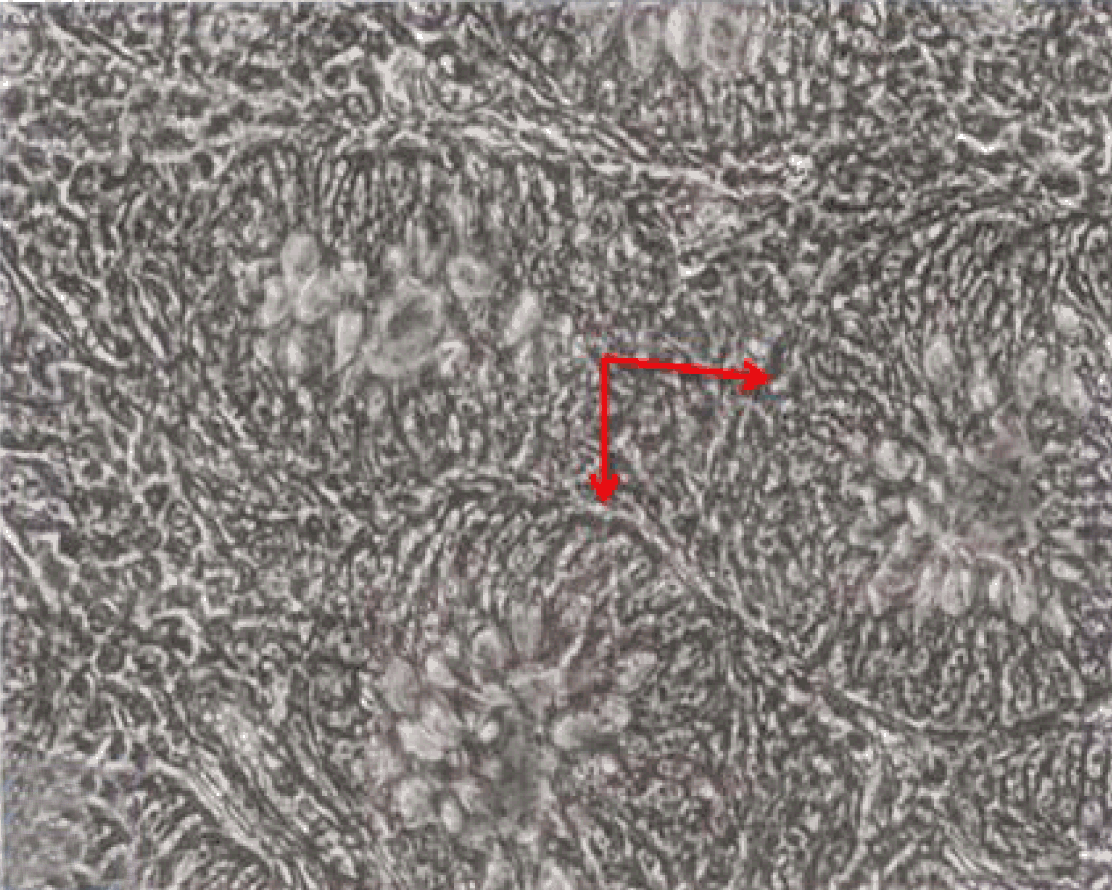
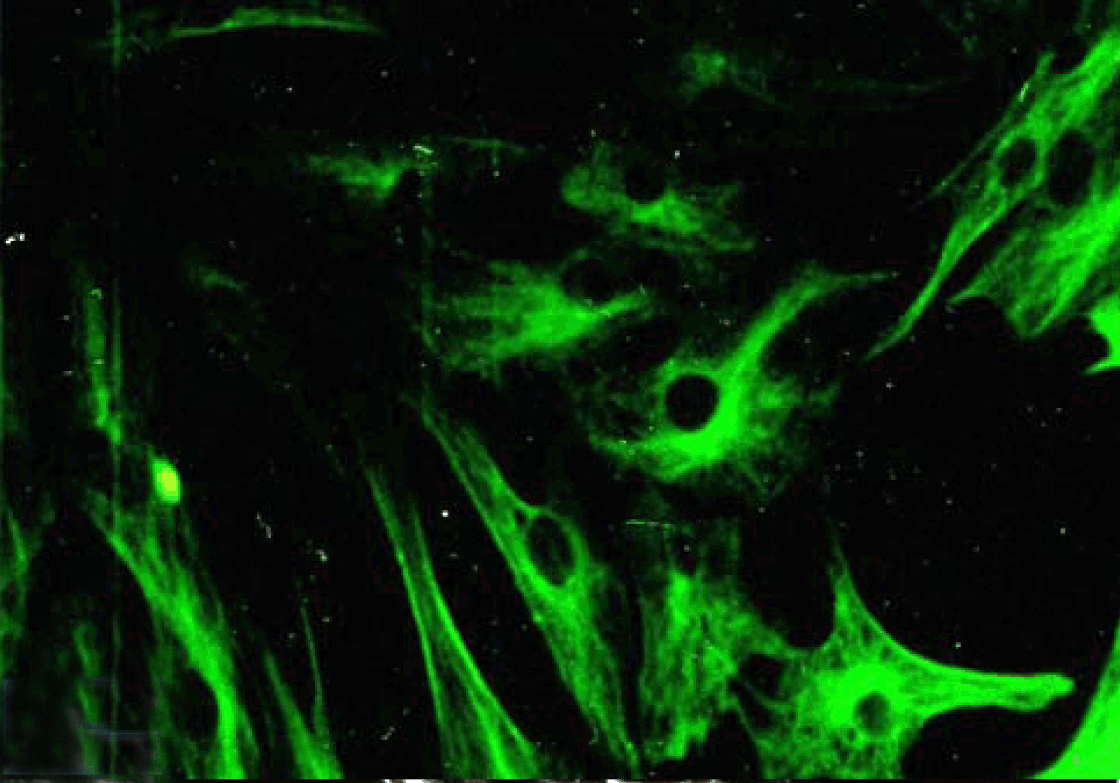
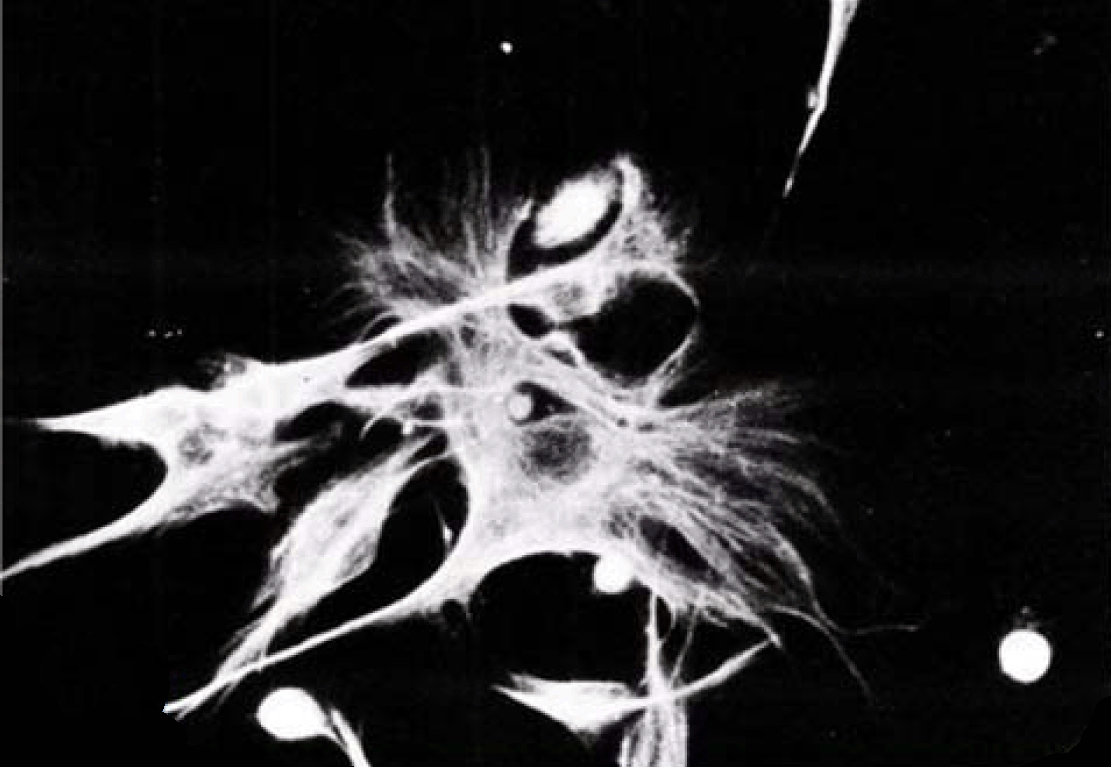
Phase contrast microscopy The non-polar human Caco-2 cells when grown as monolayer (Figure 5) or aggregates (Figure 6), showed no brush border. Inter-cellular cysts (ICC), acini-like structures, were occasionally observed within Caco-2 cells aggregates (Figure 6). ICCs were simple in early culture (Figure 7) but complex in later culture (18 days culture) (Figure 8). The Caco-2 cells gradually started to form increased number of rounded crypts-like structures. The early crypts were large and rounded at 21 days culture (Figure 9). Some were irregular and elongated (Figure 10) then became rounded. Few free cells remained in between such crypts (Figure 11). With the advancement of culture to nearly 4 weeks, the Caco-2 cells formed small nearly regular crypts (Figure 12). Such change is an indication for their spontaneous enterocytic differentiation, which is characterized with polarization of the cell layer with the formation of domes that increased in the density in older culture.
Transmission electron microscopy (TEM)
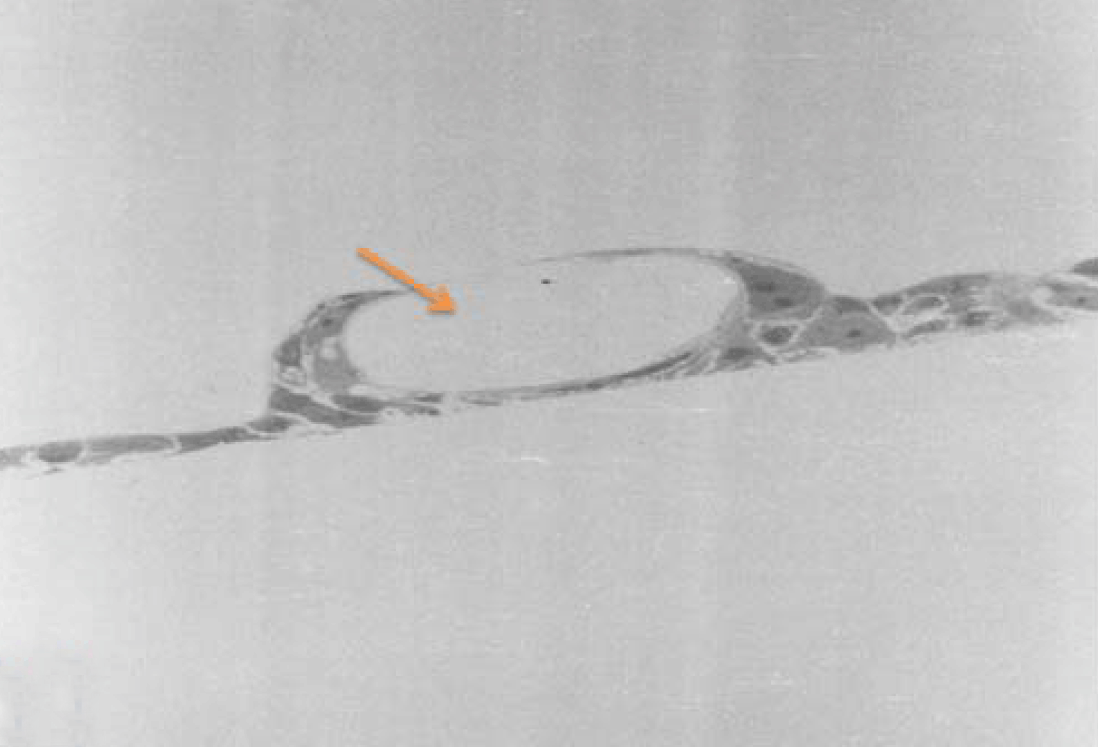
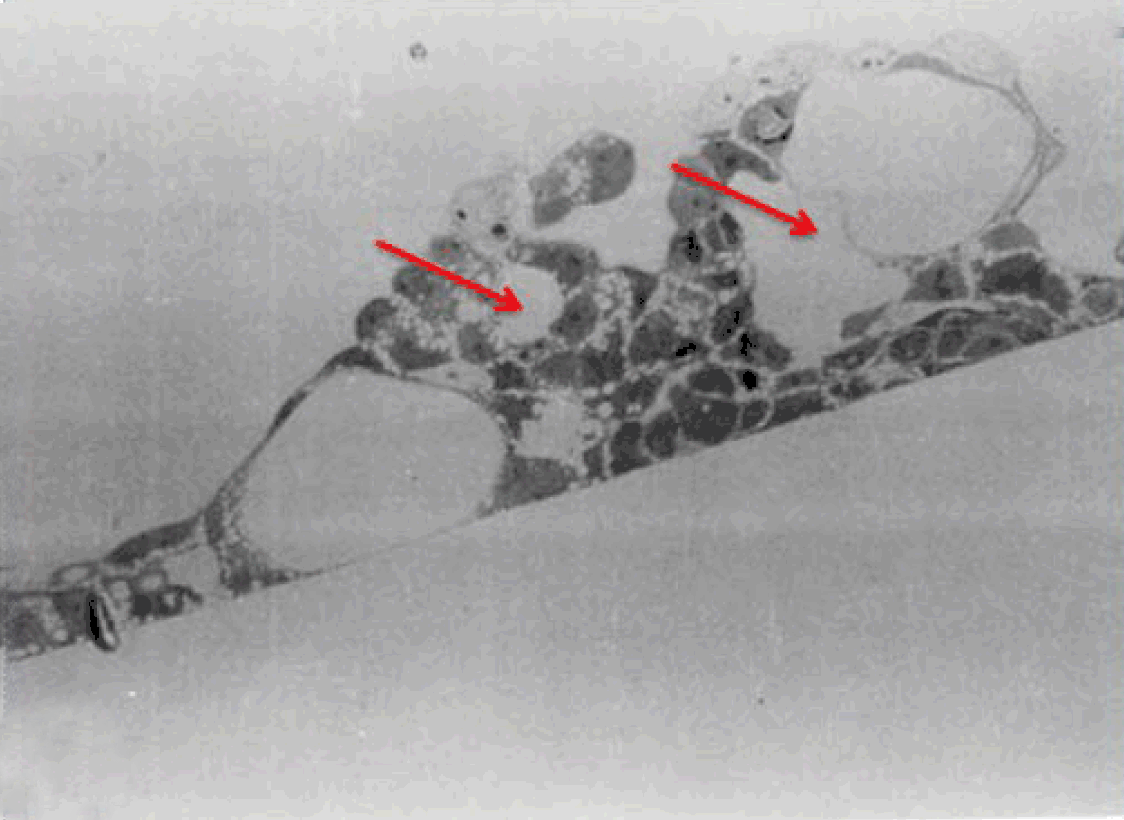
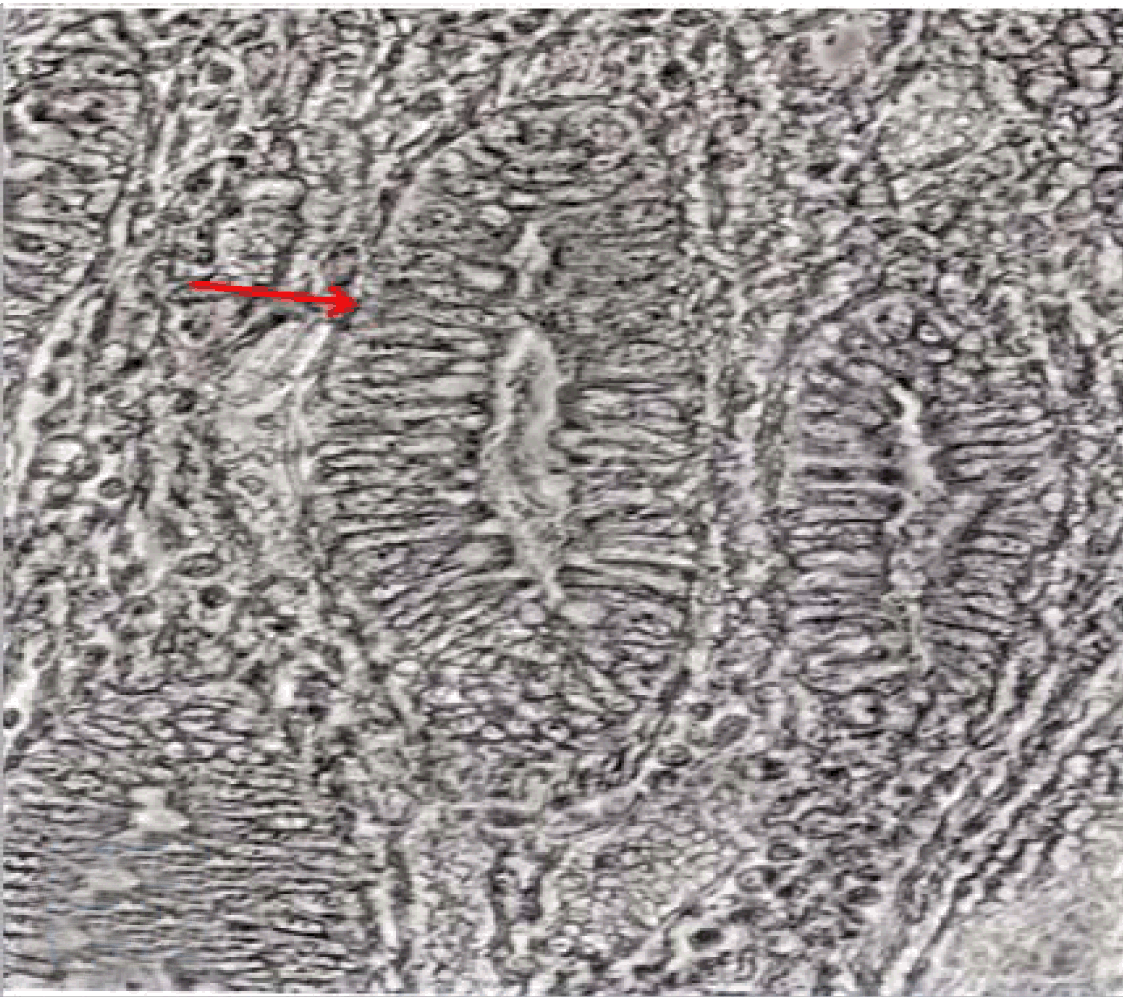
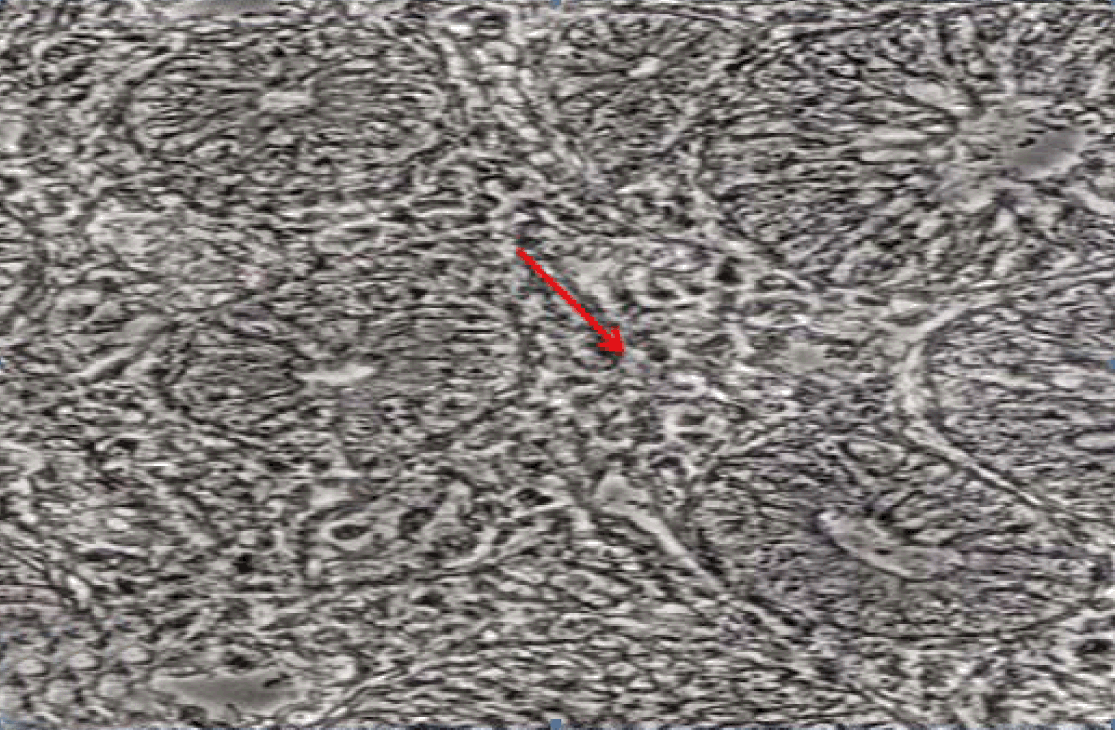
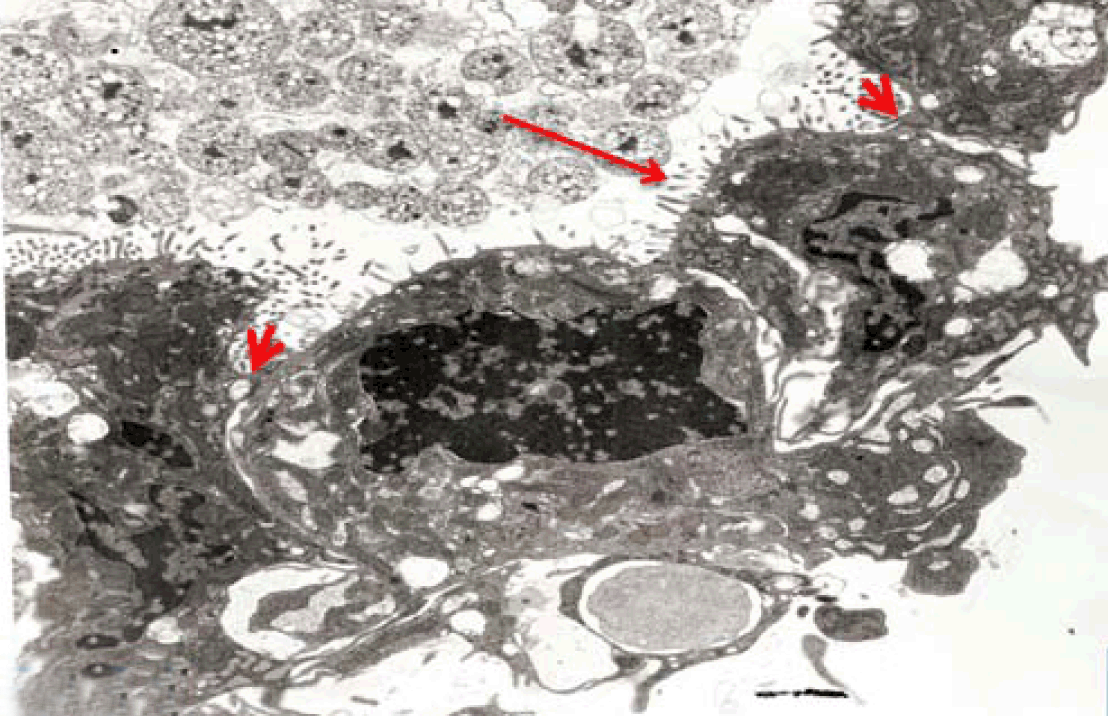
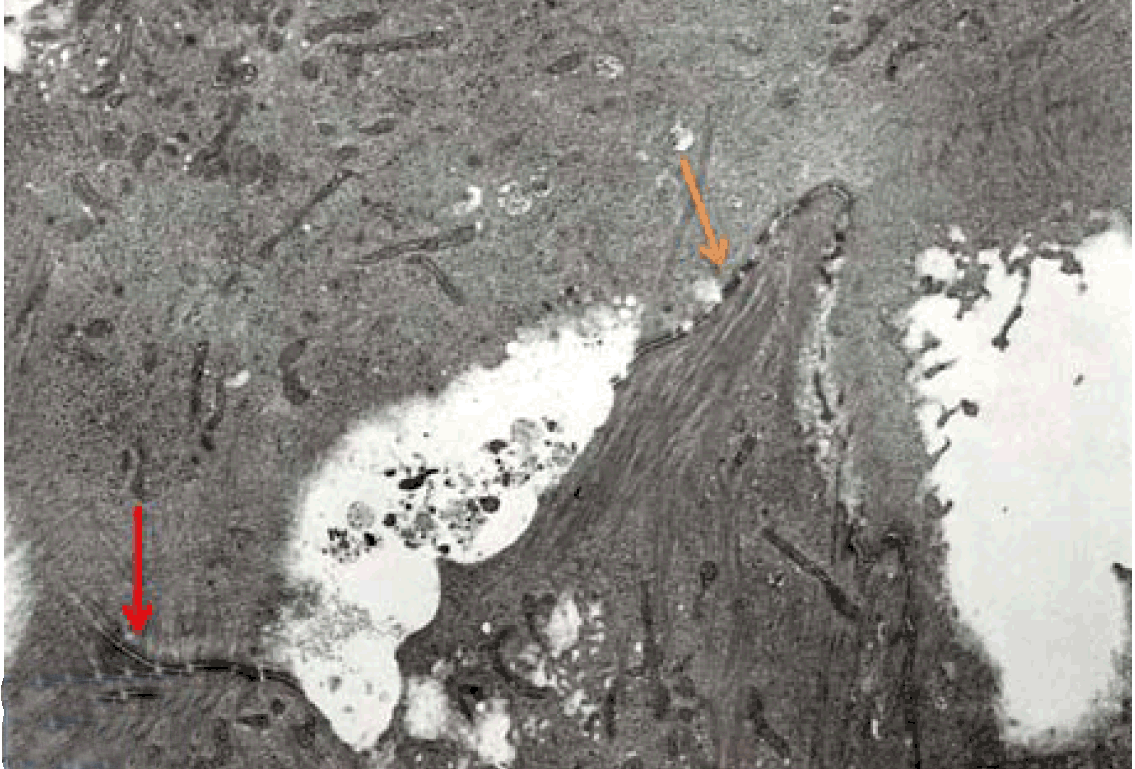
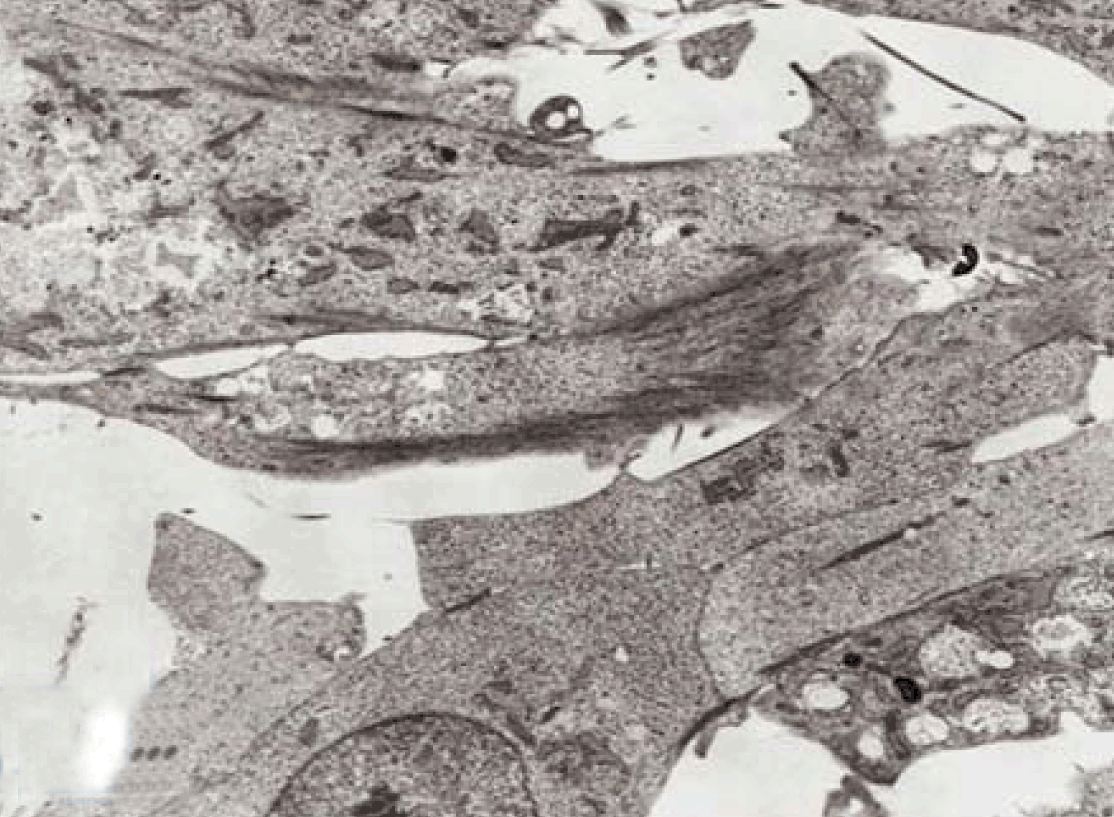
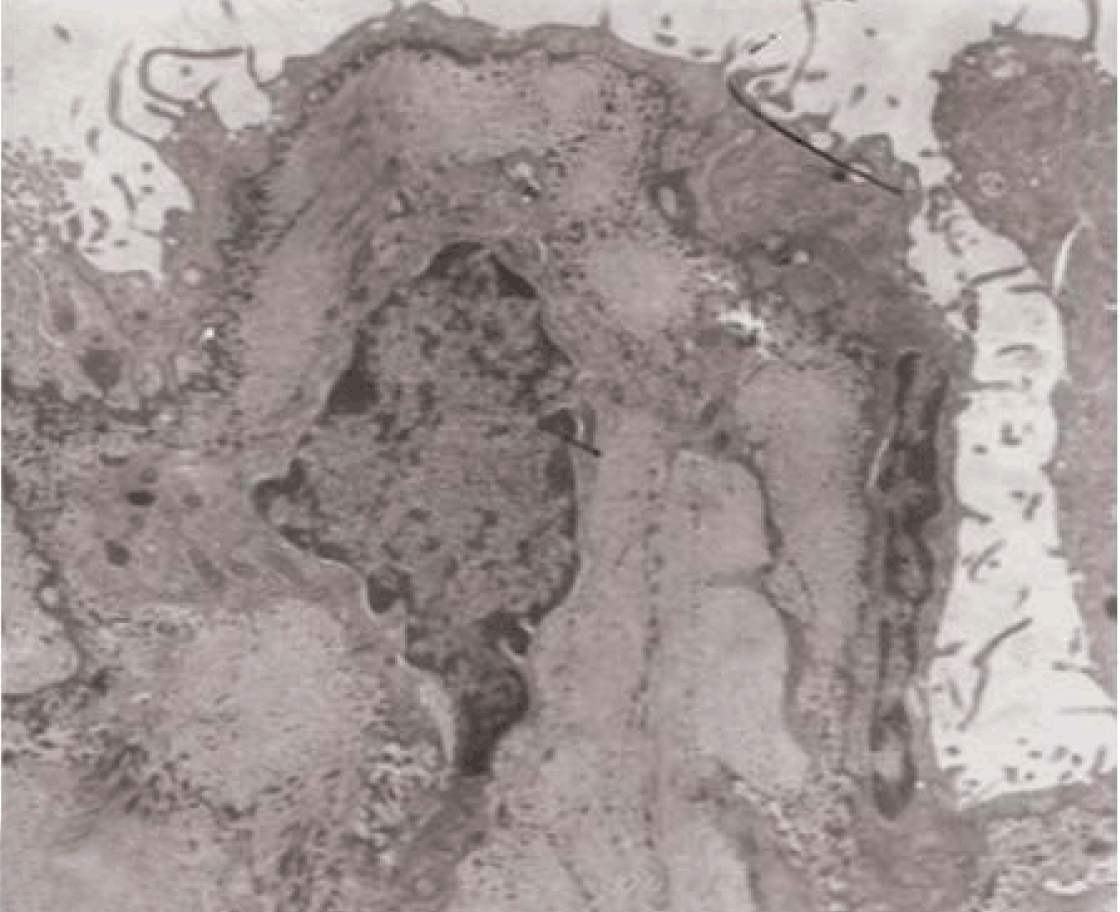
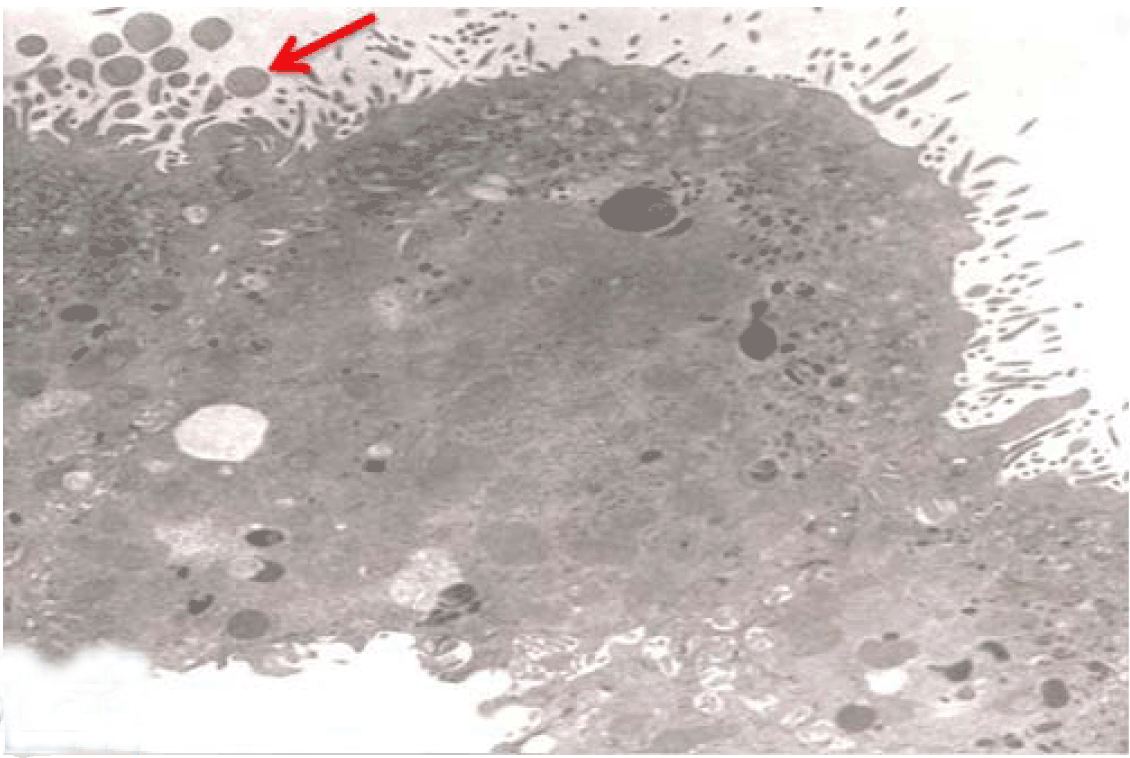
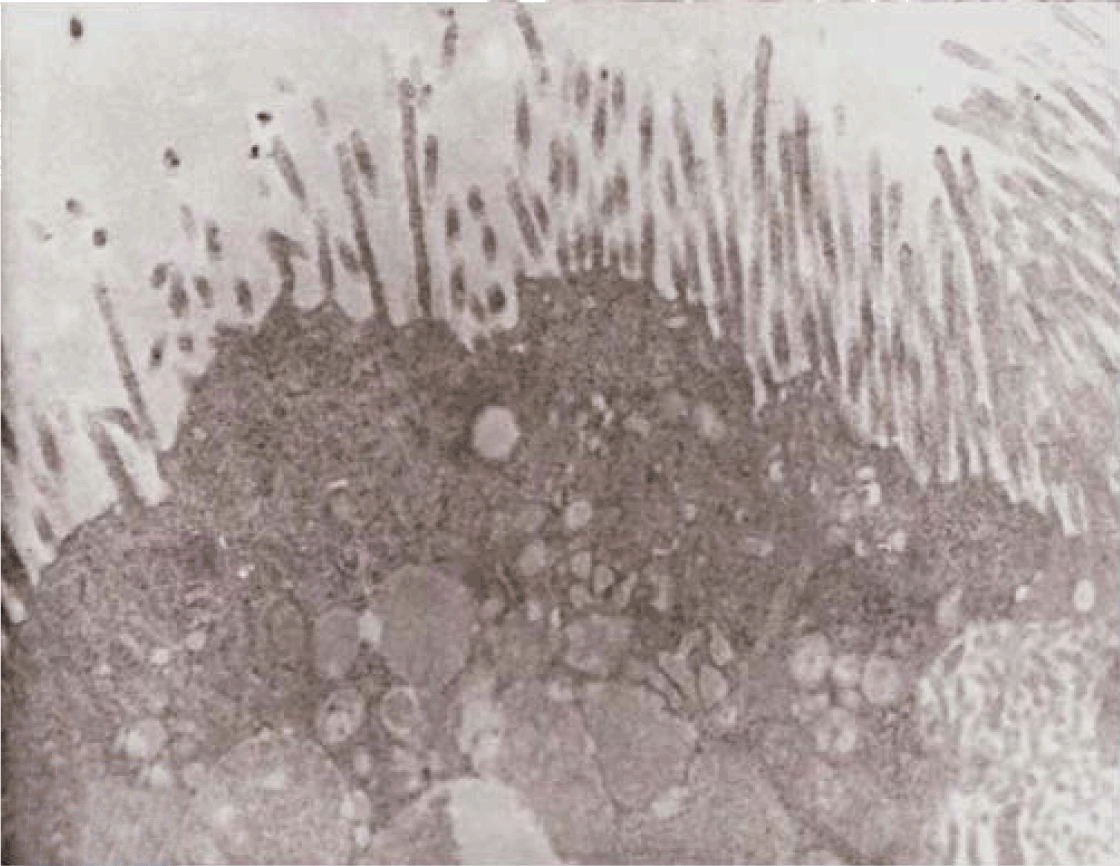
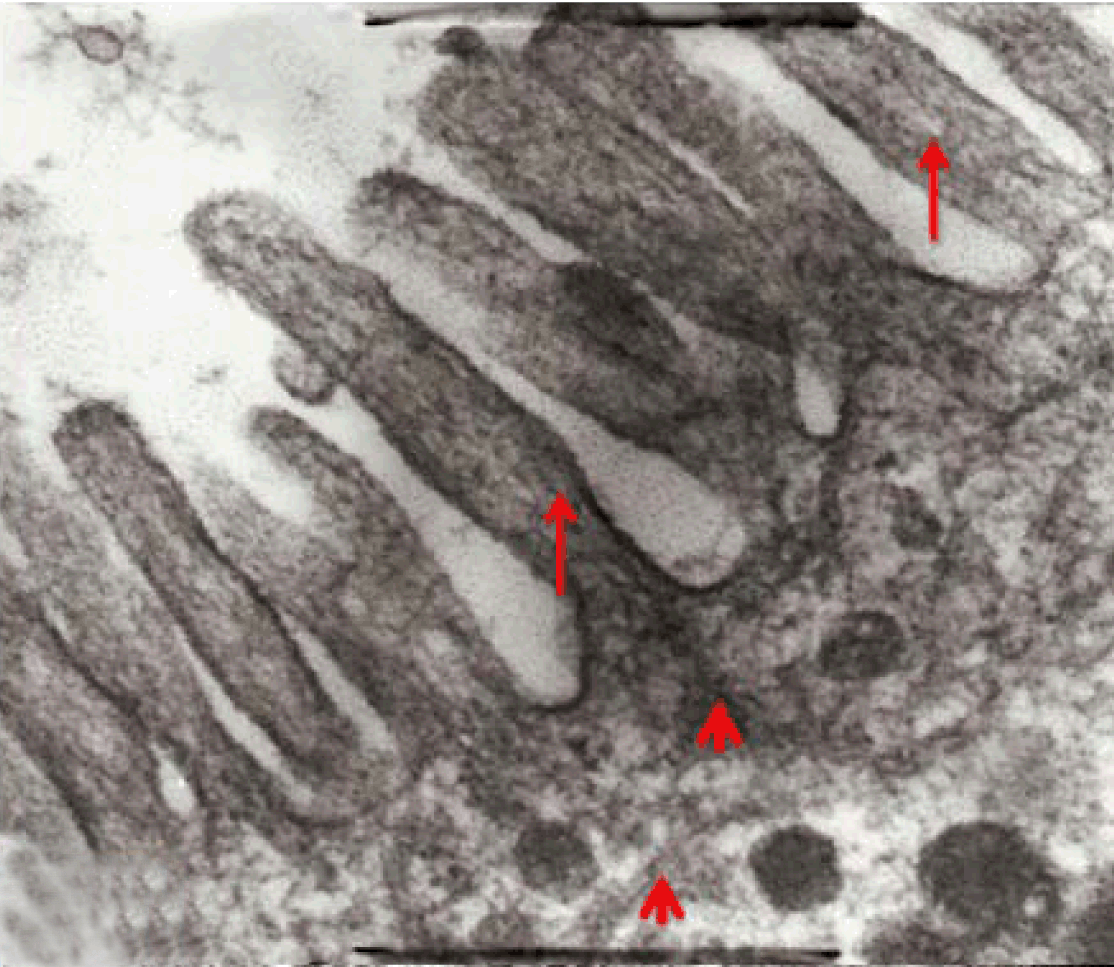
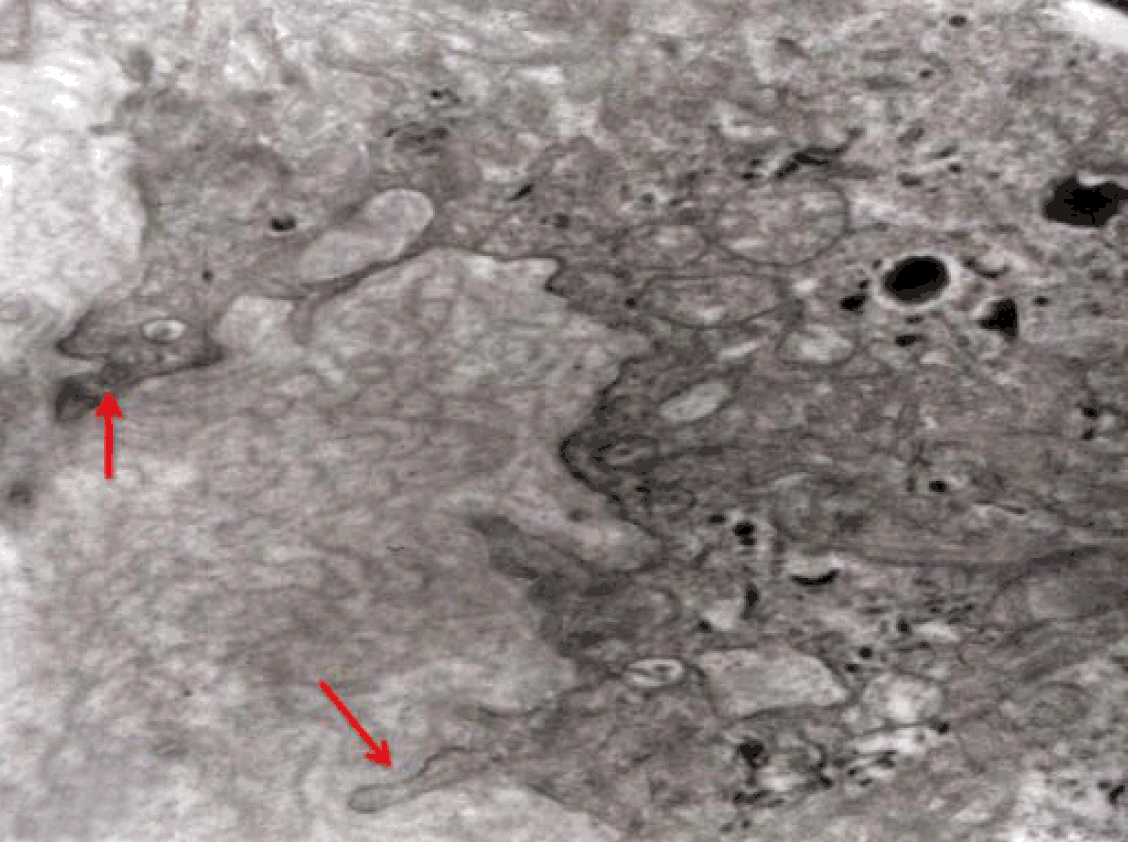
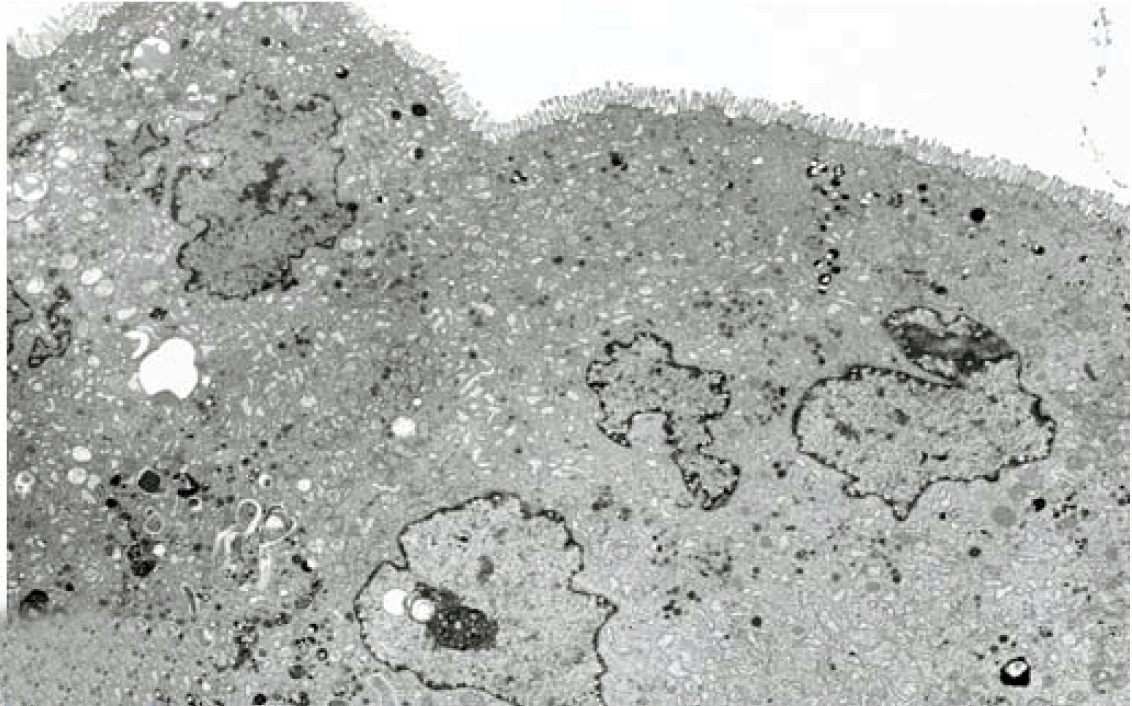
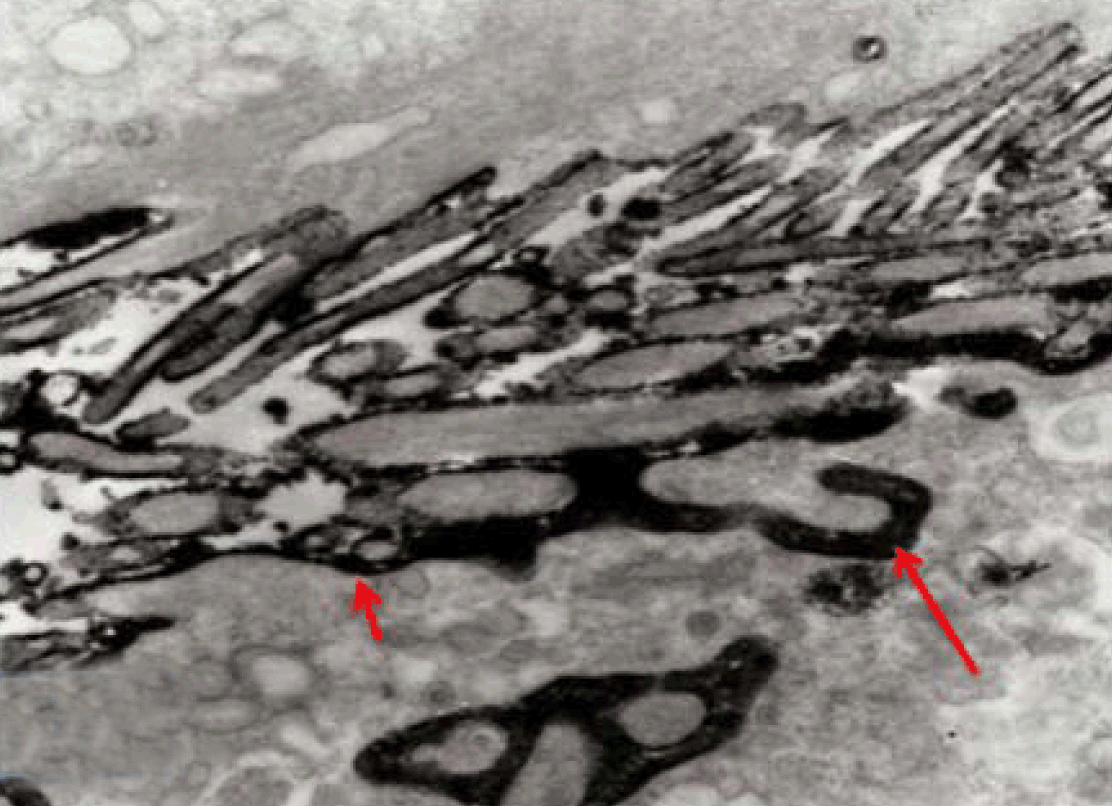
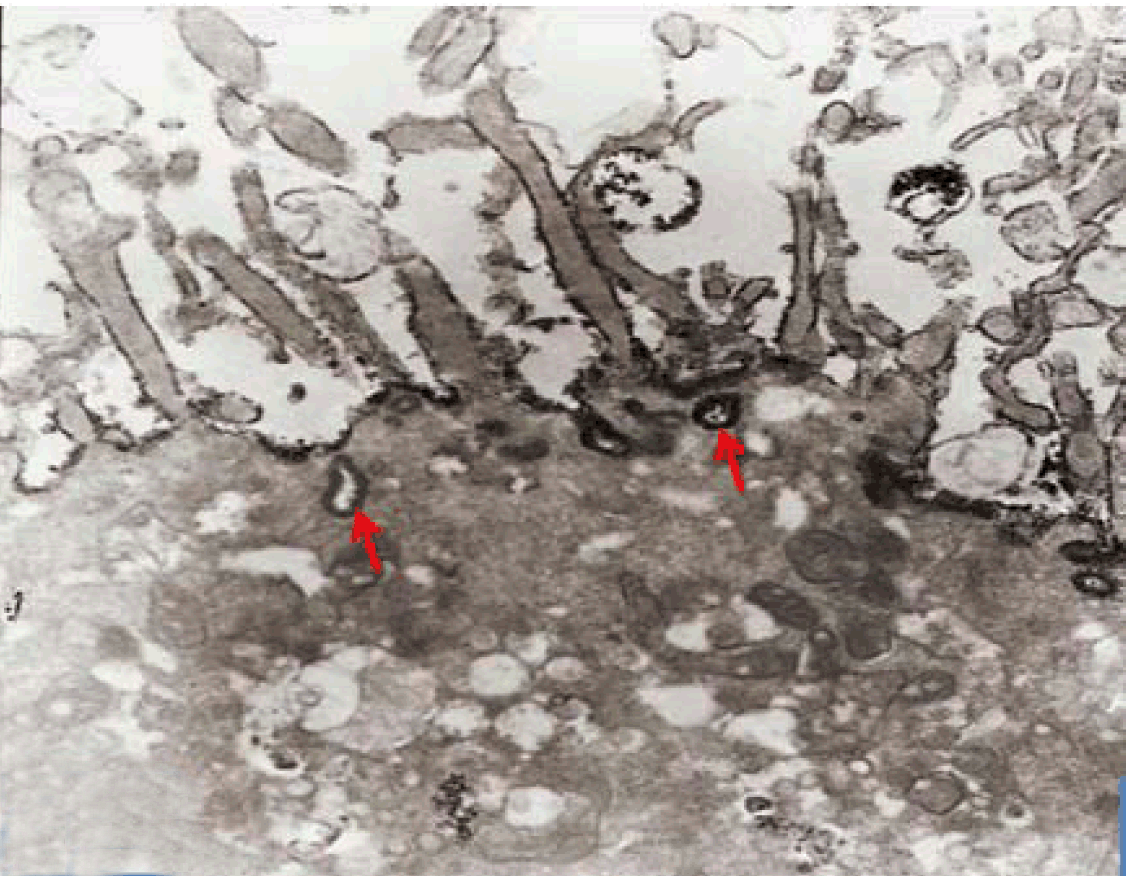
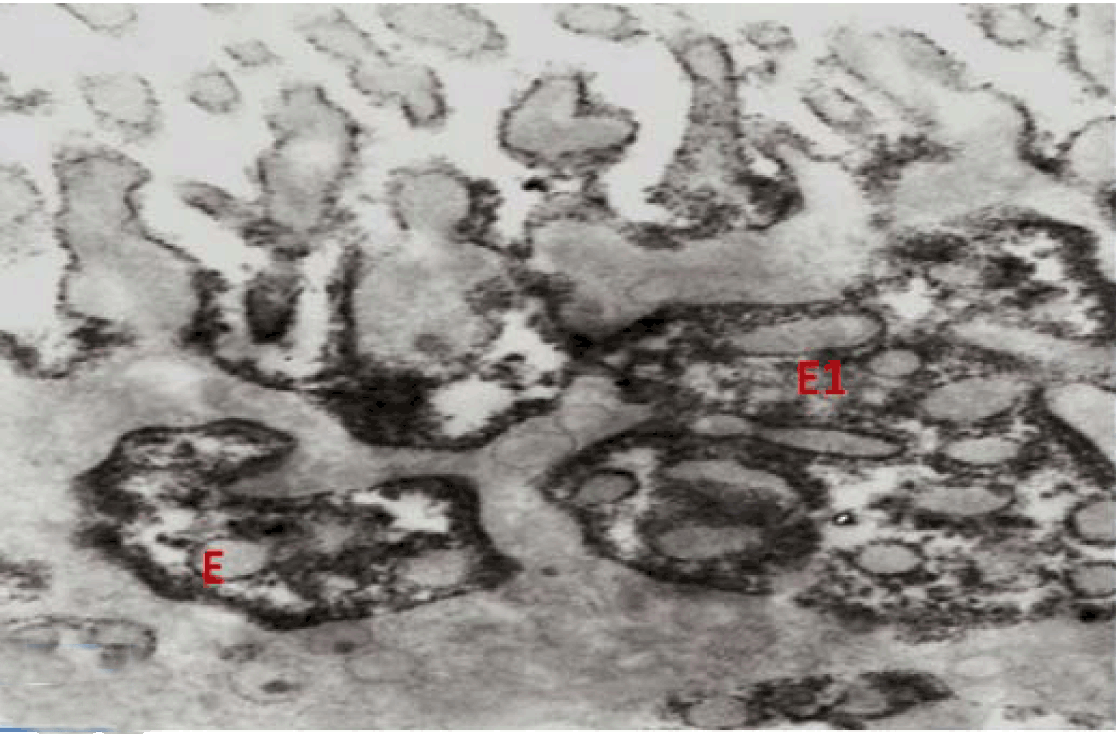
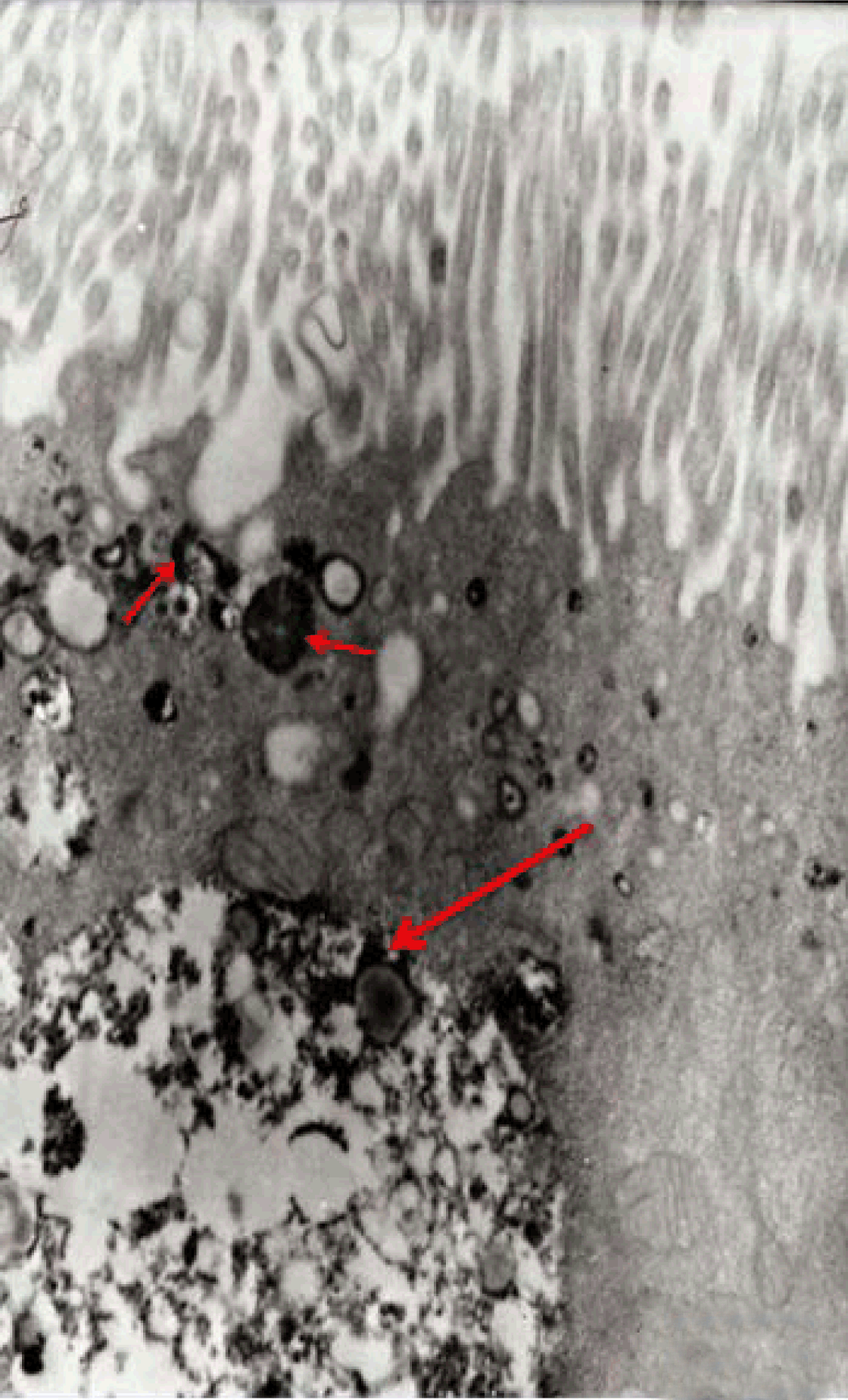
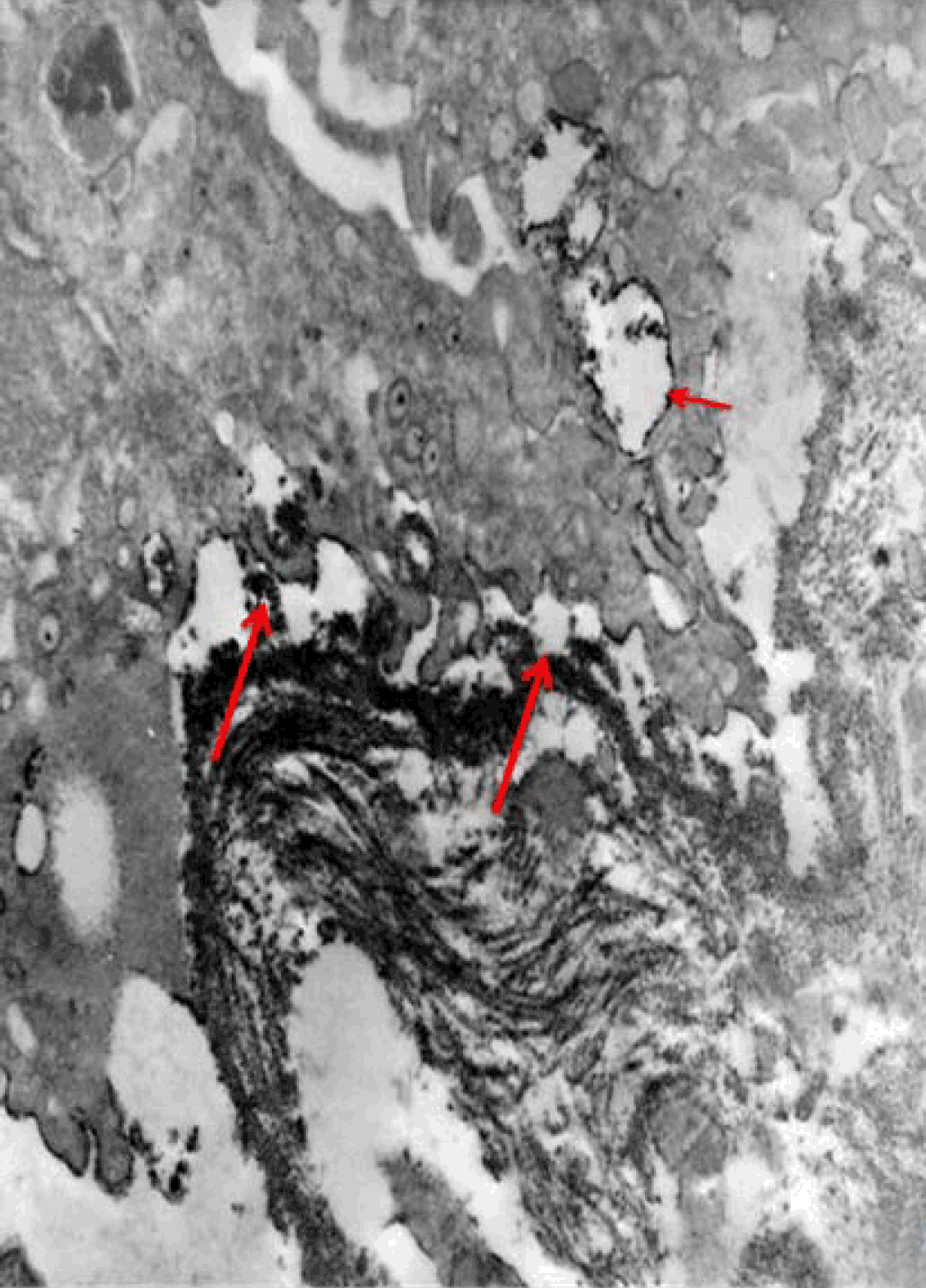
Fully polarize differentiated Caco-2 cells formed a simple cuboidal to columnar epithelium with basally-located nuclei (Figure 18). Concurrent with monolayer formation an increase in cell height, sparse microvilli with a few actin core filaments was seen. Early surfaced microvilli were short, irregular with blebs-like projections (Figure 19) then the villi became gradually lengthened and increased in density (Figure 20). Gradually the actin core bundles extended into the subjacent cytoplasm, forming the characteristic terminal web in fully differentiated Caco-2 cells (Figure 21). The cell height and microvilli- density continued to increase throughout the culture period. Coated pits and vesicles, apical tubo-vesicular system was also seen (Figures 21 and 22). Fully differentiated Caco-2 cells maintained their junctional complexes and the desmosomes. Moreover few basal projections were commonly seen (Figure 23). Frequent syncytial like growth pattern formation of fully polarize differentiated Caco-2 cells was seen with bizarre nuclear shape and numerous regular dense surface microvilli at 25 days culture (Figure 24).
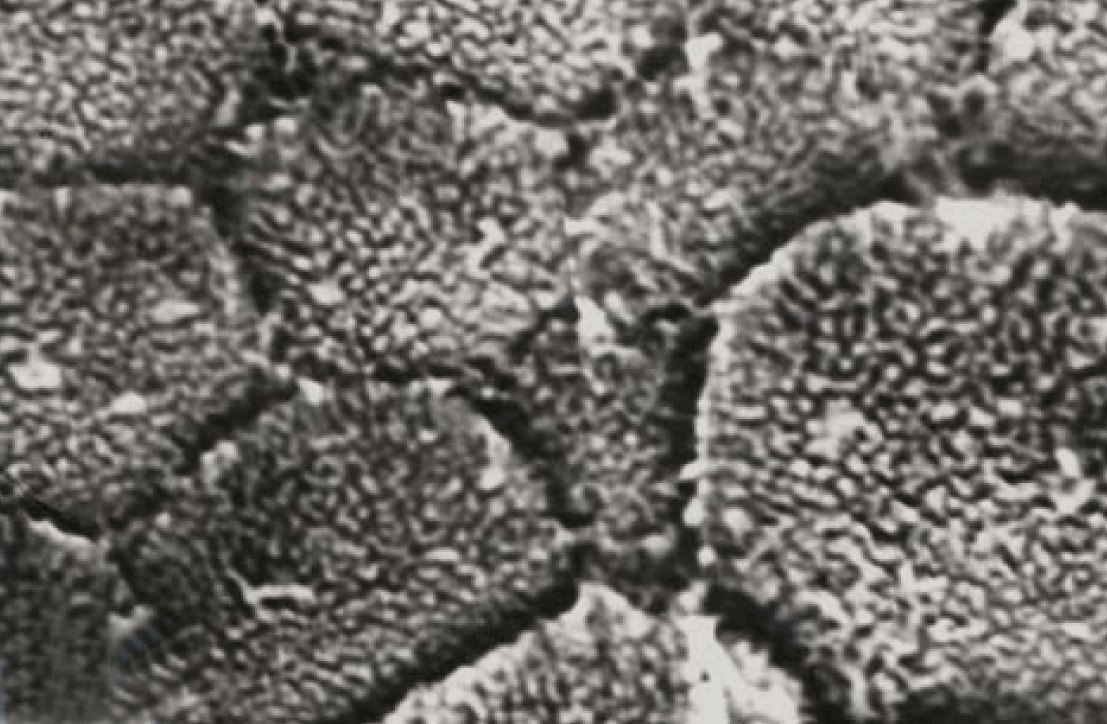
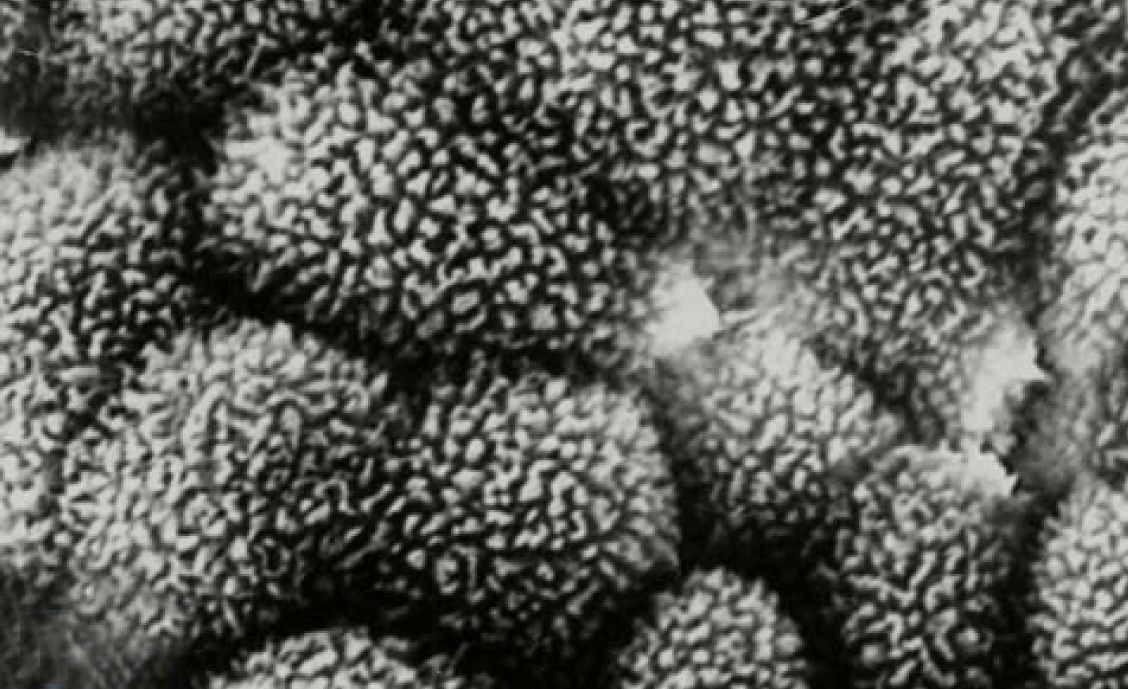
Scanning electron microscopy (SEM)
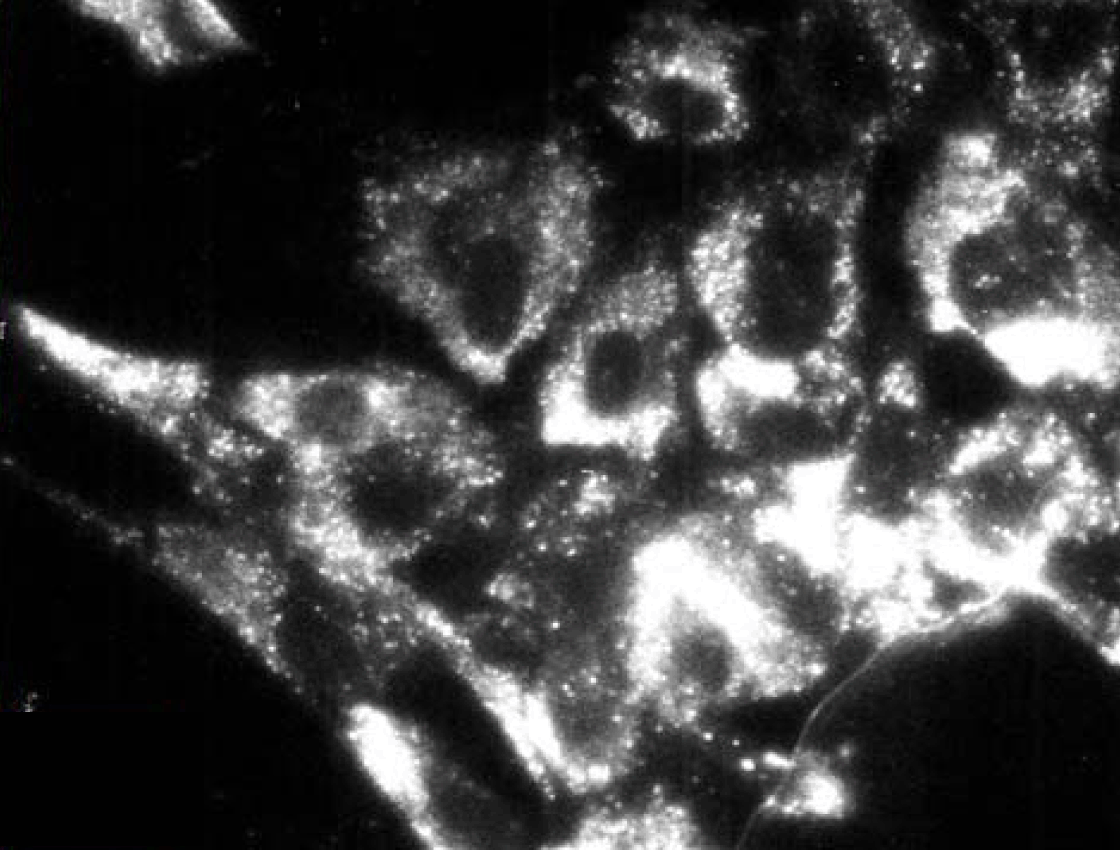
Immunofluorescence Polarized Caco-2 cell monolayer in culture stained positive with monoclonal antibody HBB specific for sucrase-isomaltase as isolated cells (Figure 32) or as aggregated acini (Figure 33).
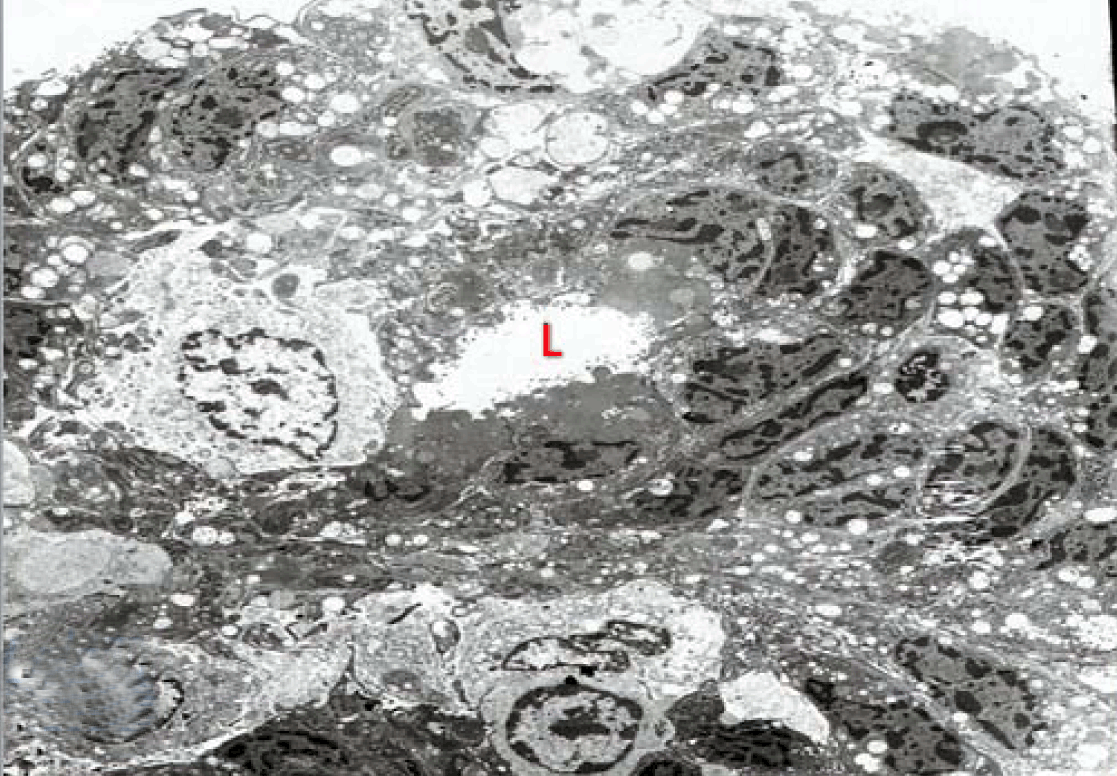
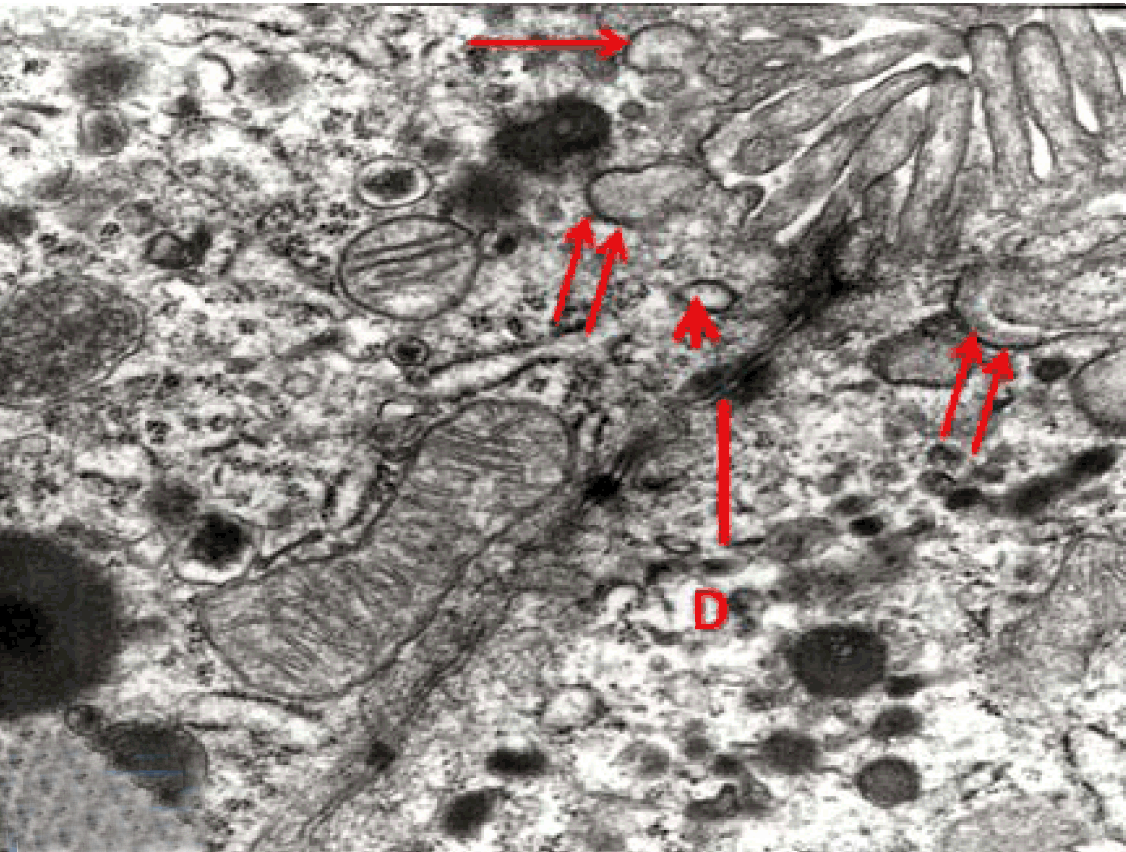
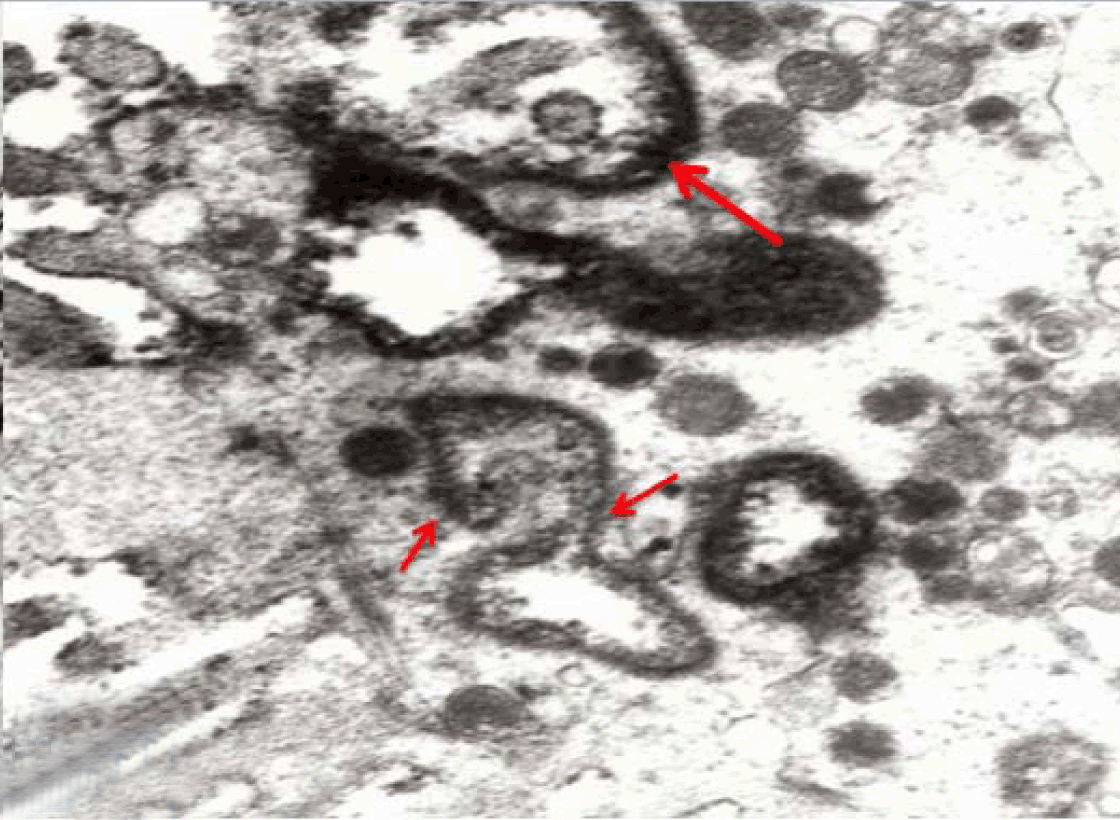
Immunoelectron microscopy
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
| ||||||
| ||||||
|
| ||||||
|
| ||||||
| ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
| ||||||
| ||||||
| ||||||
|
| ||||||
|
| ||||||
| ||||||
|
| ||||||
|
| ||||||
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
DISCUSSION
| ||||||
|
In the present study, an attempt was made to establish long term culture model of adult rabbit normal isolated intestinal epithelial cells. However, it was found that the isolated intestinal cells rapidly lost their characteristic phenotype and converted into fibroblast-like cells within one week in culture. Similar results were previously reported [2] and denote that contamination with fibroblasts led to failure of such culture model. Moreover, the mouse jejunum, when incubated for four hours, exhibited morphological deterioration of the villi with denudation of the epithelia while it retained cAMP-induced potential difference (PD) [17]. Therefore, we described an alternating in vitro cell culture system consisting of a monolayer of viable, polarized and spontaneously fully differentiated Caco-2 cells that were similar to that found in the small intestine in the absence of inducers of differentiation. In the present work, under the standard culture conditions, Caco-2 cells underwent spontaneously enterocytic differentiation both ultrastructurally and immunocytochemically. The cells developed junctional complexes, with desmosomes, very similar to adult differentiated intestinal epithelial cells. The above mentioned observations are in accordance with Hilgers et al. [11] who stated that Caco-2 cells spontaneously differentiate in culture to form confluent monolayers which both structurally and functionally resemble the small intestinal epithelium. Caco-2 cells have been used by other authors in regard to a broad spectrum of intestinal and epithelial parameters [18]. The brush border on the apical surface of enterocytes is a highly specialized structure, well-adapted for efficient digestion and nutrient transport, whilst at the same time providing a protective barrier for the intestinal mucosa [19]. The brush border is continuous with a densely ordered array of microvilli, protrusions of the plasma membrane, which are supported by actin-based microfilaments and interacting proteins and anchored in an apical network of actomyosin and intermediate filaments, the so-called terminal web [19]. In the present study Caco-2 cells in late passage of 30–40 expressed sucrase-isomaltase (SI) characteristic of epithelial enterocytes. This is supported by a similar observation [14] that the major site of expression of the enzyme sucrase-isomaltase (SI) is the brush border of the small intestine. Moreover, Caco-2, have been used extensively for analyzing the expression and biosynthesis of the enzyme sucrase-isomaltase (SI) and other brush border hydrolases [15],[16],[20]. The fully differentiated Caco-2 cells were polygonal with domed shaped surfaces bearing high density surface microvilli and well established epithelial junctional complexes including surface zonula occludes (tight junction) around the apical membrane. Gradually the actin core bundles spreads into the apical villi extending into the subjacent cytoplasm, forming the characteristic terminal web in fully differentiated Caco-2. The cell height and microvilli density continued to increase throughout the culture period. Coated pits and vesicles, apical tubo-vesicular systems were also seen. Wang et al (2017)[21] stated that dysfunction of the epithelial barrier is a hallmark of inflammatory intestinal diseases. The intestinal epithelial barrier is maintained by expression of tight junctions that connect adjacent epithelial cells and seal the para-cellular space. IL-22 is critical for the maintenance of intestinal barrier function through promoting anti-pathogen responses and regeneration of epithelial tissues in the gut utilizing polarized Caco-2 cell. In the present work, Caco-2 cells showed spontaneous differentiation in three weeks resulting in a monolayer of cells that have morphological and functional characteristics of mature enterocytes. Samak et al (2015) [10]concluded that the disruption of tight junctions and barrier dysfunction may lead to mucosal translocation of luminal toxins and pathogens to induce inflammatory process and colitis. Epithelial cells and their intercellular junctions play a primary role in the intestinal mucosal diffusion barrier to the invasion of noxious agents. Lee et al (2017) [22] indicated that Astragaloside II (AS II) can contribute to epithelial barrier repair following intestinal injury, and may offer a therapeutic avenue in treating irritable bowel disease in Caco-2 cells culture model. In the present study the uptake IgG-HRP was seen at the apical cell invagination caveolae that were frequently observed on the apical membrane at the base of the microvilli in polarized monolayer of Caco-2 cells. After 15 minutes, larger endosomes became labeled and by 30 minutes vacuolar endosomes including multivesicular bodies present deeper in the cell. After 40 min, labeled elements were widespread in the apical cytoplasm. No labeled elements occurred in the basal regions, below the nucleus. After 60 minutes, the penetration of labeled elements became very extensive reaching the basal region of the Caco-2 cells. Finally IgG-HRP was seen deep in the cell base. Such labeling is an evidence for a receptor-mediated transcytotic pathway from the apical surface to the basolateral border of Caco-2 cells. Receptor-mediated endocytosis is a specific process for cells to take up small and large molecular legends, including hormones, growth factors, enzymes, and plasma proteins [23]. Thus Receptor-mediated endocytosis, which was demonstrated in Caco-2 culture model, could be utilized for efficient drug delivery to the target cells with high expression of the receptors. For examples, transferrin receptor (TfR) and insulin receptor (IR) mediated endocytosis systems have been used for small molecules and therapeutic protein delivery [24]. Besides transport of macromolecules, endocytosis is also involved in antigen presentation, maintenance of cell polarity and regulation of cell-surface receptor expression [8]. Receptor-mediated endocytosis (RME) is driven by clathrin-coated vesicle (CCV) cycle [25]. This cycle begins with the assembly of the coat proteins; clathrin, into coated pits of apical cell membrane (ACM). Clathrin assembles into a lattice-like structure and induces inward curvature of the pit as it grows with the successive addition of coat proteins. The coated pit invaginates and eventually detaches from the ACM, forming a coated vesicle [26]. Clathrin-mediated endocytosis is the best characterized endocytic mechanism and is the predominant pathway for macromolecule uptake along epithelia [6]. Broecket al (2007) [27] suggested the involvement of the clathrin-mediated endocytic internalization route in the uptake of cholera, utilizing human intestinal Caco-2 cells. Receptor-mediated endocytosis depends on the integrity of the actin cytoskeleton and the microtubules [6]. In addition to clathrin- and caveolae-mediated endocytosis, clathrin and caveolae-independent endocytosis exists. One example of the independent endocytosis is the internalization of human IgG in Caco-2 cells. In order to understand the mechanism of the absorption of therapeutic monoclonal antibodies by the human epithelial cells, the endocytosis and internalization of a human IgG into Caco-2 was examined in the present work. Xiao and Gan (2013) [23] found that endocytosis of the human IgG into Caco-2 cells was pH, temperature, and ATP-dependent. In addition, caveolae-dependent endocytosis inhibitors, Nystatin and Indomethacin, had no significant effects on the cell association and binding of human IgG to the Caco-2 cells, indicating that the internalization is a clathrin- and caveolae-independent endocytosis [23]. Yuan et al (2017) [28] studied transport of isorhapontigenin (ISO) across Caco-2 cells and found that ISO has anti-inflammatory effect, anti-oxidation effect and anti-cancer effect. The results suggested that transport mode of ISO was mainly passive diffusion in Caco-2 cell models, and P-gp and MRP may be involved in the transport of ISO. | ||||||
|
CONCLUSION
| ||||||
|
Long term culture model of adult rabbit normal isolated intestinal epithelial cells (explants or collagenase harvested) was unstable model for intestinal barrier experiments. Caco-2 cells model could be a significant model for research on drug delivery across the intestinal epithelium. It can also be used to evaluate strategies for investigating and/or enhancing absorption, macromolecular transport by different ways across the intestinal epithelium; such as passive transcellular, independent endocytosis and carrier receptor-mediated endocytosis mechanisms. Future research is required to further characterize different active cellular efflux pumps and transporters across the enterocytes and Caco-2 cells model. | ||||||
|
REFERENCES
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Acknowledgements
The author is grateful to Prof. Hamdy M. Aly, Professor of Anatomy, Faculty of Medicine, Ain-Shams University for his valuable contribution to the research. The author would like to express his thanks to Prof. Mostafa M. El-Naggar, Professor of Anatomy, Faculty of Medicine, Jazan University for his contribution in revising the manuscript. |
|
Ethics approval and consent to participate
This study was conducted in accordance with the ethical standards of the Kingdom of Saudi Arabia prior to the beginning of work. Study documents were approved and permissions for data collection were obtained from the administration of the college. |
|
Author Contributions
Mohammed A. Akeel – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of Submission
The corresponding author is the guarantor of submission. |
|
Source of Support
None |
|
Conflict of Interest
Author declares no conflict of interest. |
|
Copyright
© 2018 Mohammed A. Akeel. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|