| Table of Contents |  |
|
Case Report
| ||||||
| An unusual cause of pancreatic head mass: Primary pancreatic tuberculosis | ||||||
| V. YaminiChitra1, K. N. Paramesh2 | ||||||
|
1MS (General Surgery), DNB, MCH (Surgical Gastroenterology), Associate Professor, Dept of Surgical Gastroenterology and Bariatric Centre, Vydehi Institute of Medical Sciences and Research Centre, Bangalore, Karnataka, India.
2MS (General Surgery), (DNB Surgical Gastroenterology), Senior Resident, Dept of Surgical Gastroenterology and Bariatric Centre, Vydehi Institute of Medical Sciences and Research Centre, Bangalore, Karnataka, India. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article: |
| YaminiChitra V, Paramesh KN. An unusual cause of pancreatic head mass: Primary pancreatic tuberculosis. Edorium J Gastroenterol 2015;2:7–12. |
|
Abstract
|
|
Introduction:
In tuberculosis, abdominal involvement occurs in 12% of cases with miliary tuberculosis (TB) and isolated abdominal involvement occurs in 20% of extrapulmonary TB. Isolated tuberculous involvement of pancreas is extremely rare accounting for 5% of these cases. The aim of this case report is to present a rare case of isolated tuberculosis of pancreas in a young nonalcoholic, immunocompetent patient with vague symptomatology and no clinical signs.
Case Report: A 22-year-old male patient presented with vague abdominal pain and weight loss of two months duration. Evaluation revealed a pancreatic head mass with biliary obstruction, endoscopic ultrasound guided fine needle aspiration (EUS–FNA) was inconclusive and preoperative diagnosis of pancreatic neoplasm was made. Patient underwent exploratory laparotomy, it was decided that resection was not possible and therefore a palliative biliary and gastric bypass with biopsy was done. Postoperative histology showed tuberculosis of pancreatic tissue and peripancreatic nodes. The patient was started on antitubercular drugs and improved well. Conclusion: In a patient with pancreatic mass, a high index of suspicion, use of EUS-FNA with acid-fast bacilli (AFB) staining, TB-PCR and culture techniques help diagnose pancreatic TB and avoid unnecessary surgeries in this totally curable condition. | |
|
Keywords:
Pancreatic head mass, Tuberculosis, EUS-FNA, TB-PCR
| |
|
Introduction
| ||||||
|
Tuberculosis (TB) is a common disease in developing countries and incidence is increasing with the rise of acquired immunodeficiency syndrome and with the use of immunosuppressant drugs [1] [2] . Abdominal involvement occurs in 12% of cases with miliary TB and isolated abdominal involvement occurs in 20% of extra pulmonary TB. The most common sites of involvement in the abdomen are the mesentery, small bowel, peritoneum, liver, and spleen. Isolated tuberculosis involvement of pancreas is extremely rare accounting for 5% of these cases [1]. Herein, we report a case of pancreatic head mass with biliary obstruction suspected to be a pancreatic neoplasm but postoperatively proved as pancreatic TB. | ||||||
|
Case Report
| ||||||
|
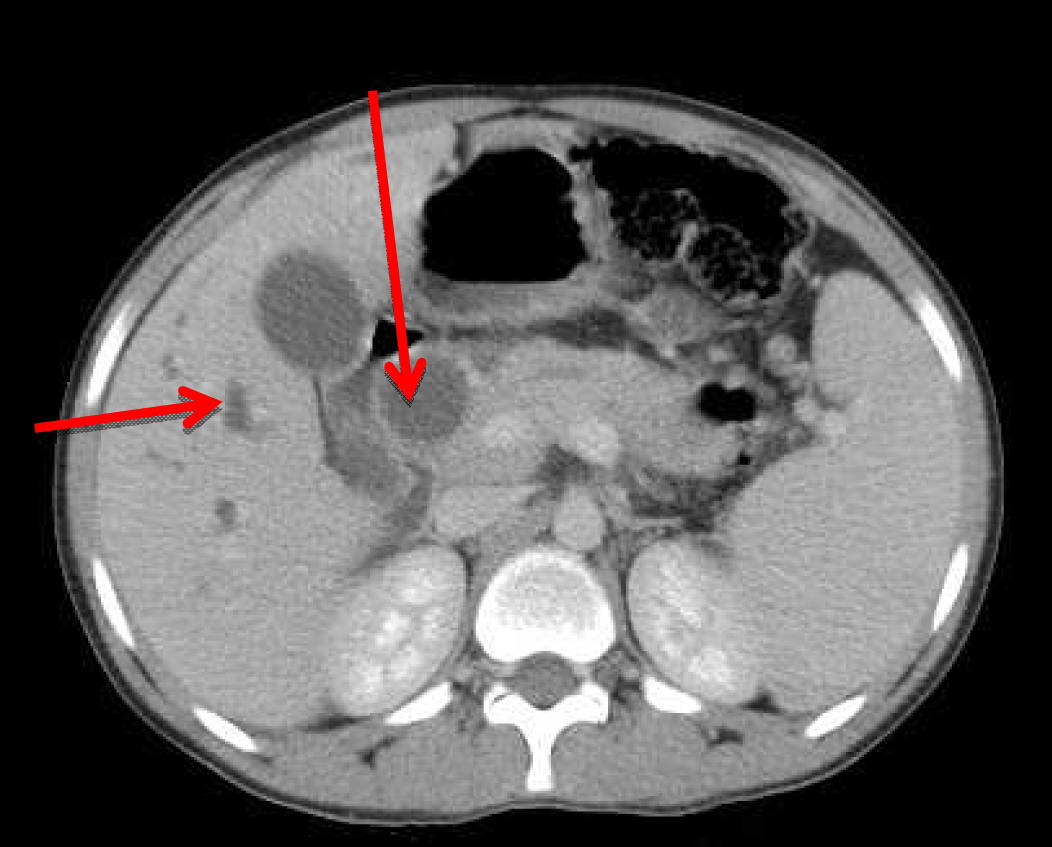
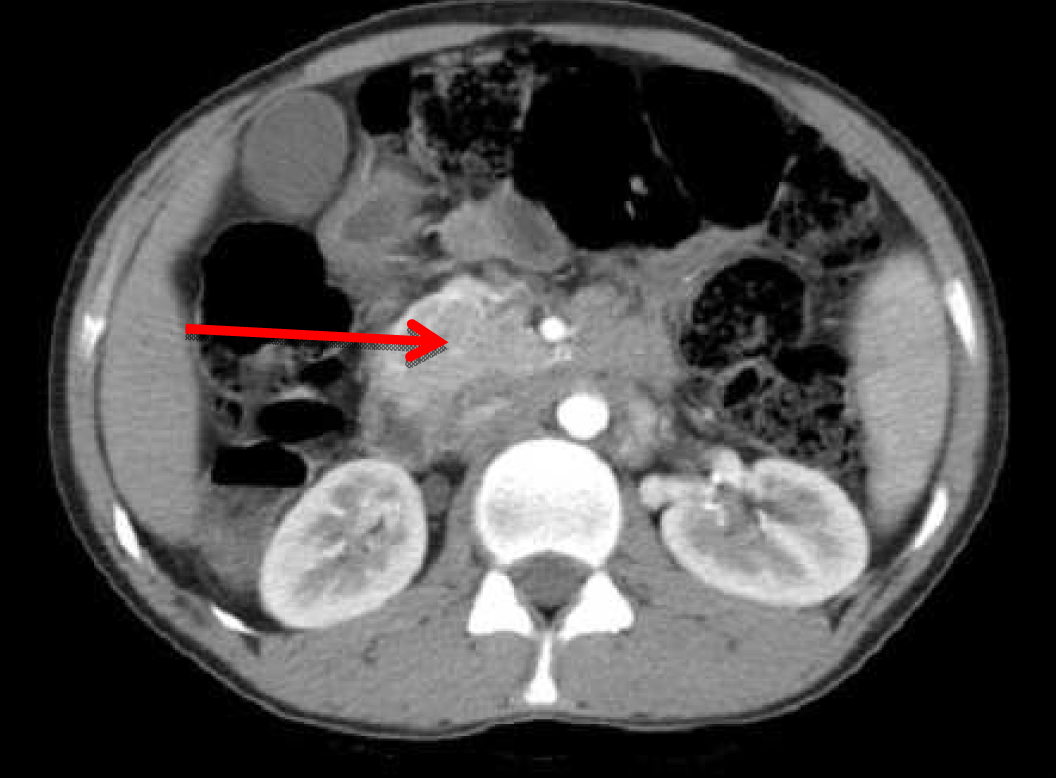
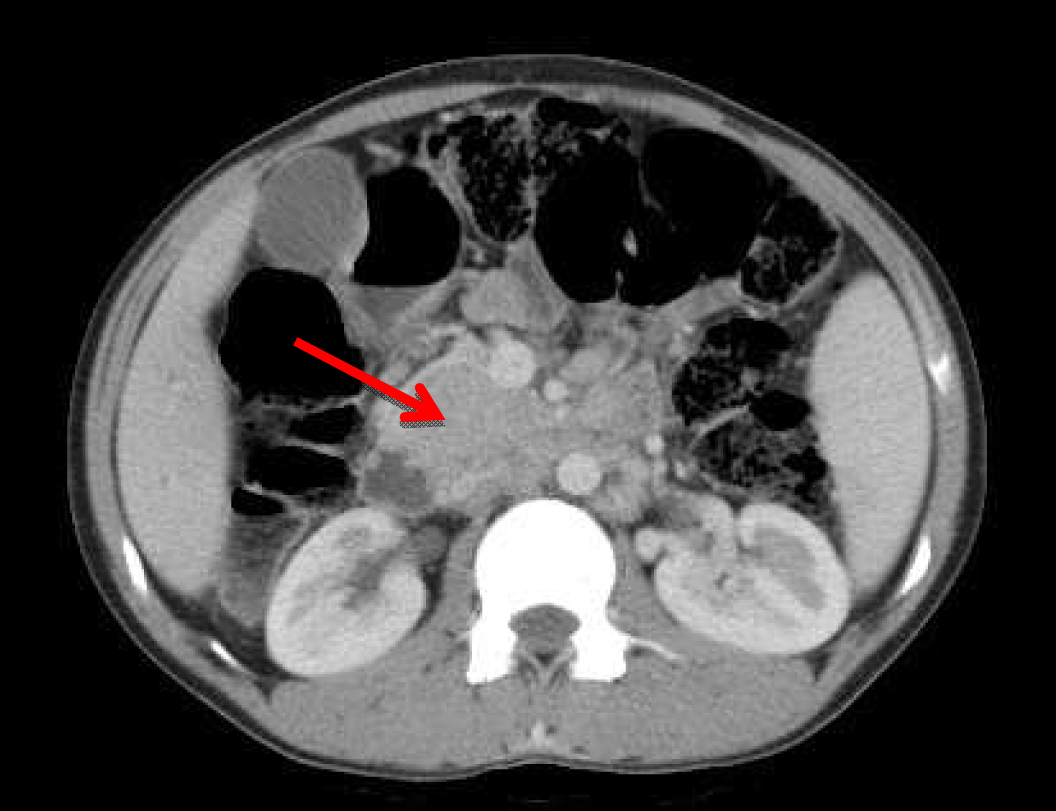
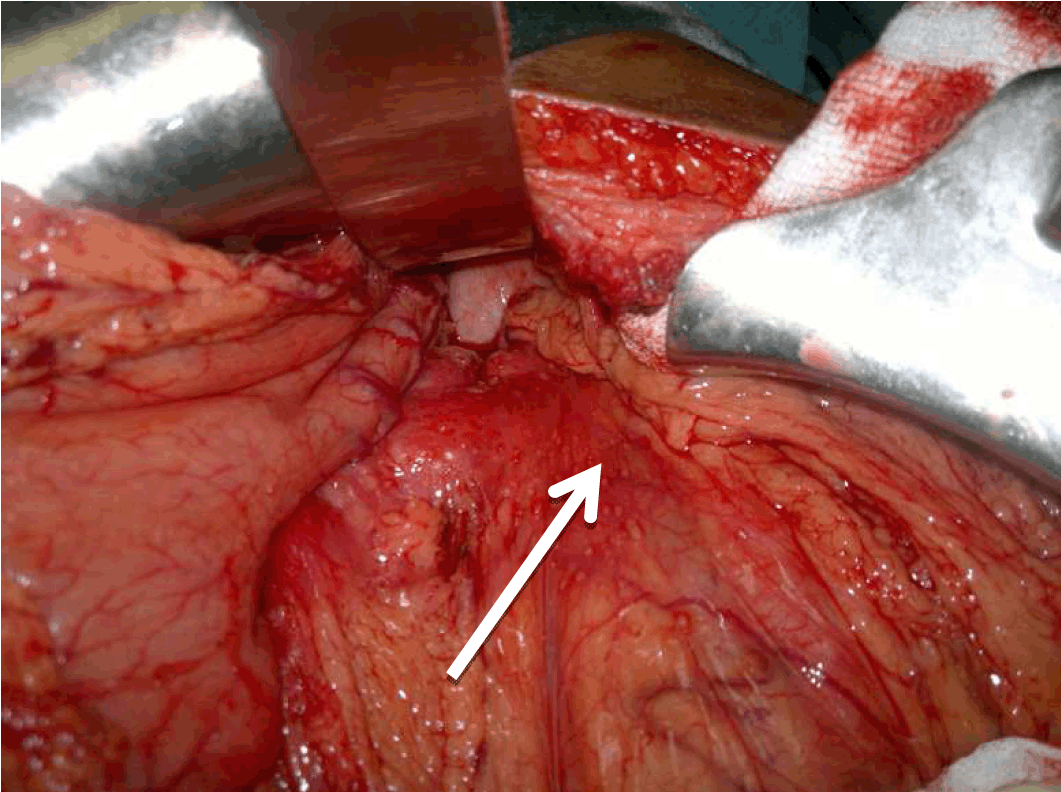
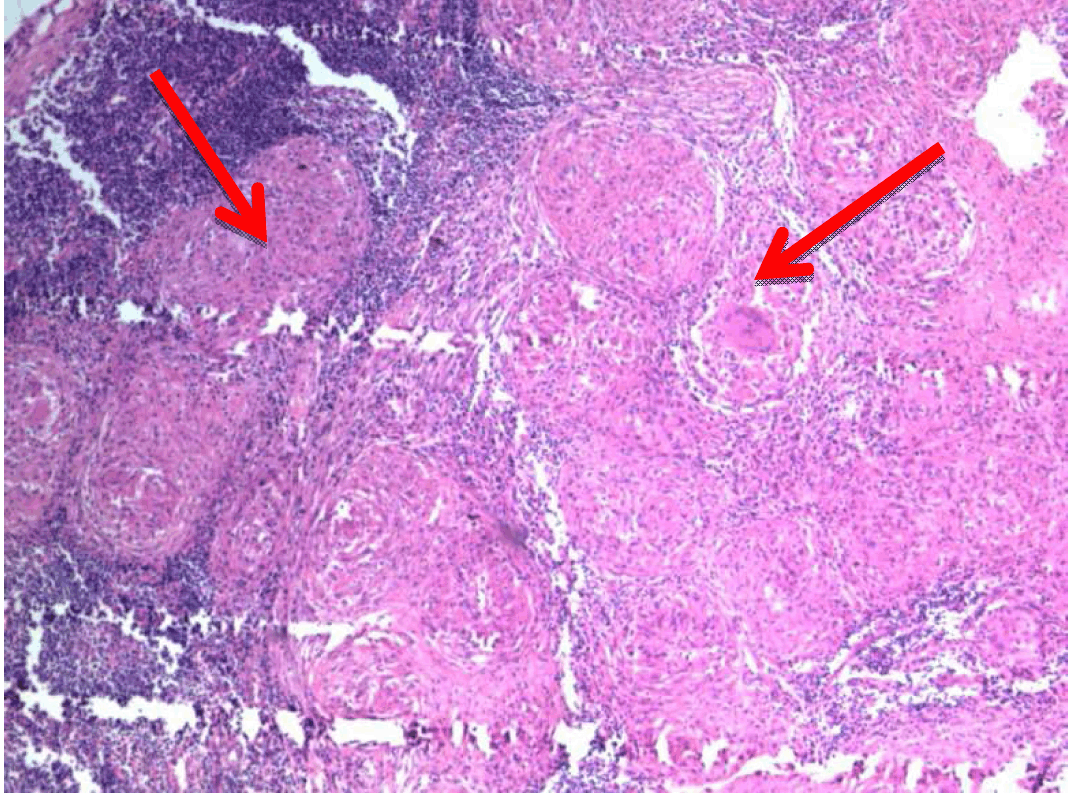
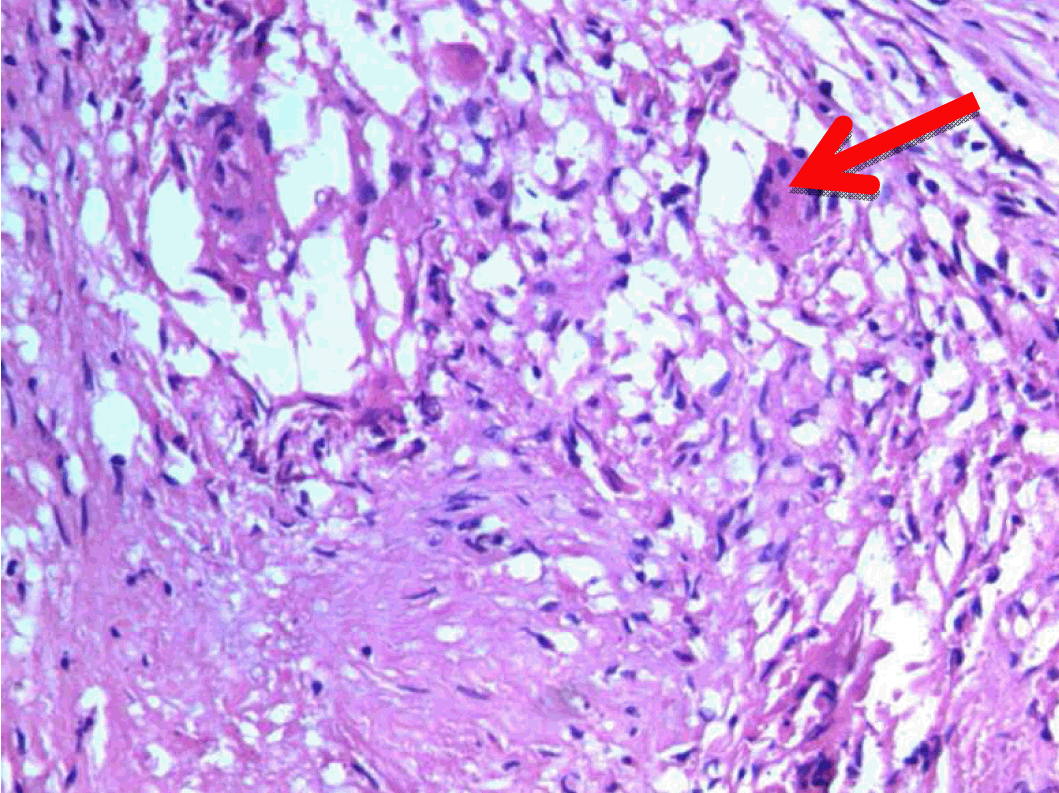
A 22-year-old male presented with dull aching pain abdomen and significant weight loss of two months duration and no significant past history. Clinical examination was normal. Liver function test revealed total bilirubin 1.5 mg%, ALP -602 IU/L, AST-96 IU/L, ALT-106 IU/L. Chest X-ray was normal and HIV was negative. Ultrasound (USG) of abdomen showed hypoechoic mass in the head of the pancreas, with dilated intrahepatic biliary radical (IHBR) and common bile duct (CBD). Contrast-enhanced computed tomography (CECT) revealed a mass lesion measuring 30.2x47.9x62.3 mm in the head of pancreas (Figure 1) with a mildly dilated pancreatic duct, IHBR and CBD dilated, mass enhancing in the arterial phase (Figure 2), isodense with rest of pancreas in venous phase (Figure 3). Peripancreatic and periportal lymph nodes were enlarged. Endoscopic ultrasound (EUS) showed a pancreatic head mass with multiple peripancreatic lymph nodes. EUS-FNA was negative for malignancy. A tumor in head of pancreas was suspected and patient was subsequently taken for pancreaticoduodenectomy. During the operation it was observed that there was a mass in the head of the pancreas and an enlarged and edematous body and tail. There were firm and enlarged lymph nodes measuring 1x1 cm around the celiac axis and in the peripancreatic region. Peritoneum of mesocolon showed few scattered lesions suspicious of peritoneal deposits/tubercles over pancreatic surface (Figure 4). Liver was normal, dilated common bile duct was found. Rest of the abdomen was normal. Intraoperative fine needle aspiration cytology (FNAC) of head mass, body of pancreas and imprint cytology of peripancreatic lymph node was taken which revealed features of small cells, suggesting neuroendocrine tumor. Since portal vein could not be dissected free at level of neck of pancreas and body and tail were enlarged, oedematous, the resection procedure was abandoned, patient underwent palliative biliary and gastric bypass along with pancreatic head biopsy, excision of a peripancreatic node and the portion of the mesocolon for histology. Postoperative period was uneventful. Histopathological examination revealed epithelioid granuloma with Langhans type of giant cells in pancreatic tissue, peripancreatic lymph nodes and mesocolic tissue (Figure 5) and (Figure 6). Based on pathological examination, diagnosis of pancreatic tuberculosis was made and patient was started on anti-tubercular drugs for six months. On follow-up after two years, patient improved well and gained weight and is totally asymptomatic. | ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
Discussion
| ||||||
|
Tuberculosis is caused by Mycobacterium tuberculosis, an acid-fast bacillus and is a common disease worldwide. In miliary TB involving abdomen, lung involvement is always present [3], on contrary to extrapulmonary TB where there is no lung involvement. In isolated pancreatic TB, focal involvement is more common, first in the head, followed by body and tail. Diffuse involvement is rare [4]. It can present as discrete pancreatic cystic or solid masses mimicking malignancy [4] [5] [6] [7], acute or chronic pancreatitis or as pancreatic abscesses [3]. Peripancreatic nodal involvement occurs in most of the cases. Infection of the pancreas is thought to occur by direct extension to the organ via lymphatic or hematogenous spread [5] [8] or following reactivation of previous abdominal TB [2]. Clinical presentation of pancreatic TB is often insidious, with nonspecific constitutional symptoms of abdominal pain (75–100%), anorexia, weight loss (69%), malaise, weakness (64%), fever and night sweats (50%) [3] [9]. Gastrointestinal bleed is possible due to splenic vein thrombosis [3], portal vein thrombosis [6] or extrahepatic portal vein obstruction due to mass effect. Laboratory investigations reveal hypertransamina-semia and elevated alkaline phosphatase values in greater than 50% of cases [3] . The noninvasive diagnostic techniques for pancreatic TB rely mainly on ultrasonography and CT scan of abdomen. Ultrasonography features include focally or diffusely enlarged pancreas with areas of hypoechogenicity and cysts [9] [10]. Peripancreatic and mesenteric lymphadenopathy, ascites, splenic and hepatic focal lesions may also coexist [9]. Findings on CT scan include hypodense nodules or diffuse enlargement of pancreas, calcifications, cystic and multiloculated lesions, enlarged peripancreatic and periportal nodes with peripheral ring enhancement [9] [11] [12]. In CT, pancreatic adenocarcinoma, pancreatic lymphomas may have similar appearance like pancreatic TB and cystadenocarcinoma and pseudocyst can mimic cystic form of pancreatic TB. Primary pancreatic lymphoma will also present with predominant symptoms of abdominal pain and weight loss [13] and jaundice is rare but lymphoma occurs in older age group (57.5 years). CT findings in lymphoma localized to pancreatic head show hypodense lesion which shows poor enhancement with iv contrast [13] [14]. The CBD and pancreatic duct (PD) may be dilated or encased but PD dilatation is minimal [14]. H. Lin et al. [13] have diagnosed 4 out of 6 cases of primary pancreatic lymphoma only after pancreatic resection due to rarity of the condition and difficulty in diagnosis. Enhancing lesions in arterial phase of CT abdomen have been usually due to neuroendocrine tumors or metastatic lesions to the pancreas [15]. Enhancement in arterial phase in pancreatic tuberculosis is not reported so far in literature. So the suspicion of tuberculosis was not considered in this patient. Sonthalia et al. have suggested that CBD and PD are rarely dilated in TB pancreas in contrast to adenocarcinoma of pancreas [11] but in our patient CBD was grossly dilated and pancreatic duct was mildly dilated increasing the suspicion of pancreatic neoplasm. On magnetic resonance imaging (MRI), T1-weighted fat suppressed images, pancreatic tuberculous lesion appears hypointense, and on T2-weighted images it shows heterogeneous signal intensities [10]. FDG PET-CT scan also shows increased uptake in these lesions increasing the diagnostic dilemma [16]. Bile cytology or endoscopic retrograde cholangiopancreatography (ERCP) has low diagnostic yield [16]. Endoscopic ultrasound guided fine needle aspiration (EUS–FNA) is the diagnostic modality of choice in suspicious pancreatic masses with a sensitivity of 85–90% [16] On-site cytology increases diagnostic yield by 15%, appropriate cultures can be done the same time, repeated multiple needle passes can be avoided by prompt diagnosis [17] [18]. Intraoperative FNAC or biopsy can provide microscopic features of caseating granulomatous inflammation. Cytology reveals aggregates of epitheloid histiocytes, plasma cells and lymphocytes. Acid-fast bacilli may also be found but only in about 60% of cases. Cultures of mycobacteria are highly specific but less sensitive and takes six weeks to grow [17]. Recent Polymerase chain reaction (PCR) can detect mycobacterium tuberculosis DNA and it is most specific even when other special stains are negative [3]. Chatterjee et al. [19] have reported three cases of young patients with predominant symptoms of weight loss and abdominal pain and EUS showed pancreatic lesions with large peripancreatic nodes with areas of central necrosis. FNA showed granuloma in all their three cases and in all Ziehl-Neelsen stain and culture was done. In 2 out of 3 cases staining was negative but culture was positive and in one both were negative. So we have concluded that any patient with suspicion of pancreatic TB to do Ziehl -Neelsen staining, AFB culture and PCR assay on EUS FNA specimens to increase diagnostic yield. Gerke et al. [20] has suggested that if granulomas are found on EUS-FNA, staining for AFB and AFB cultures need to be done, to differentiate tuberculosis from other granulomatous conditions like sarcoidosis or histoplasmosis. AFB cultures and staining increase the diagnostic accuracy in these circumstances. So though there are no specific guidelines on when to do AFB staining or culture, a high index of suspicion in young patients with predominant symptoms of abdominal pain, weight loss, history of tuberculosis elsewhere in the body, a complex mass in pancreas with necrotic peripancreatic nodes, a good yield on EUS -FNA confirmed by rapid onsite cytology examination and presence of granulomas on cytology should prompt the clinician to do Ziehl-Neelsen staining, AFB cultures and PCR to rule out TB. Only laparotomy has been conclusive in some cases [9], diagnosis has been established after pancreatic resections in some [6]. In our patient neither acid-fast bacilli (AFB) staining nor culture was done on EUS- FNA specimen nor on biopsied specimen as tuberculosis was not suspected at that time. Intraoperative cytology was also misleading in our case. Management of pancreatic TB is based on anti tubercular treatment for 6–12 months, with complete response clinically and radiologically [9]. Revised National TB Control Programme (RNTCP) - TBC India guidelines [21] consistent with WHO and the International Union Against Tuberculosis and Lung Disease (IUATLD) guidelines suggest that abdominal TB is considered seriously ill and treated as category 1 and four drugs isoniazid, rifampicin, pyrazinamide and ethambutol to be given for two months and isoniazid, rifampicin for four months. In clinically suspected relapse, where patient is not improving after two months of antituberculous treatment or culture or histological proven relapse, total duration of therapy is eight months including streptomycin. The response to treatment varies from 87–97% [21]. No definitive guidelines are available in literature regarding role of reimaging. Singh et al. [22] have suggested radiological follow-up to document regression of the mass but time intervals are not exactly specified. They have suggested that if radiological improvement does not occur even after anti-tubercular treatment, the mass has to be resected for histological confirmation. But there is no data available on co-existing pancreatic carcinoma with tuberculosis. Jethwani et al. [23] have also suggested monitoring patient's weight, erythrocyte sedimentation rate and ultrasound or CT scan for imaging but not specified as to when to do and when total radiological resolution occurs. Xia et al. [12] have done a follow-up CT scan in 11 of 16 patients and found complete resolution in all, though time was variable between 78–186 days with a mean of 132 days. So we conclude that a repeat imaging at the end of antitubercular treatment and thereafter at regular intervals will help us monitor and understand the natural process of the disease. | ||||||
|
Conclusion
| ||||||
|
Tuberculosis (TB) of pancreas though rare and varied in presentation should always be in the list of differential diagnoses in a patient with pancreatic mass, especially in the developing countries. It can also occur as isolated pancreatic TB in immunocompetent individuals. High index of suspicion, use of EUS-FNA with acid-fast baccilli staining, PCR-DNA and culture techniques help diagnose pancreatic TB and avoid unnecessary surgeries in this totally curable condition. | ||||||
|
Acknowledgements
| ||||||
|
We would like to acknowledge and thank Dr. Rohini the radiologist and Dr. Summana Devanand, the pathologist who reviewed the CT films and histopathology slides respectively and helped us. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
V. YaminiChitra – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published K. N. Paramesh – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2015 V. YaminiChitra et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|